Microtechnology-based methods for organoid models
- PMID: 34567686
- PMCID: PMC8433138
- DOI: 10.1038/s41378-020-00185-3
Microtechnology-based methods for organoid models
Abstract
Innovations in biomaterials and stem cell technology have allowed for the emergence of novel three-dimensional (3D) tissue-like structures known as organoids and spheroids. As a result, compared to conventional 2D cell culture and animal models, these complex 3D structures have improved the accuracy and facilitated in vitro investigations of human diseases, human development, and personalized medical treatment. Due to the rapid progress of this field, numerous spheroid and organoid production methodologies have been published. However, many of the current spheroid and organoid production techniques are limited by complexity, throughput, and reproducibility. Microfabricated and microscale platforms (e.g., microfluidics and microprinting) have shown promise to address some of the current limitations in both organoid and spheroid generation. Microfabricated and microfluidic devices have been shown to improve nutrient delivery and exchange and have allowed for the arrayed production of size-controlled culture areas that yield more uniform organoids and spheroids for a higher throughput at a lower cost. In this review, we discuss the most recent production methods, challenges currently faced in organoid and spheroid production, and microfabricated and microfluidic applications for improving spheroid and organoid generation. Specifically, we focus on how microfabrication methods and devices such as lithography, microcontact printing, and microfluidic delivery systems can advance organoid and spheroid applications in medicine.
Keywords: Bionanoelectronics; Electrical and electronic engineering.
© The Author(s) 2020.
Conflict of interest statement
Conflict of interestThe authors declare that they have no conflict of interest.
Figures

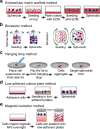
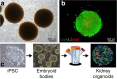

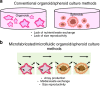


References
-
- Breslin S, O’Driscoll L. Three-dimensional cell culture: the missing link in drug discovery. Drug Discov. Today. 2013;18:240–249. - PubMed
-
- Clevers H. Modeling development and disease with organoids. Cell. 2016;165:1586–1597. - PubMed
-
- Lazzari G, Couvreur P, Mura S. Multicellular tumor spheroids: a relevant 3D model for the: In vitro preclinical investigation of polymer nanomedicines. Polym. Chem. 2017;8:4947–4969.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
