3D-Printed electrochemical sensor-integrated transwell systems
- PMID: 34567709
- PMCID: PMC8433167
- DOI: 10.1038/s41378-020-00208-z
3D-Printed electrochemical sensor-integrated transwell systems
Abstract
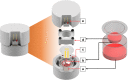
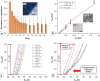
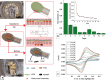
This work presents a 3D-printed, modular, electrochemical sensor-integrated transwell system for monitoring cellular and molecular events in situ without sample extraction or microfluidics-assisted downstream omics. Simple additive manufacturing techniques such as 3D printing, shadow masking, and molding are used to fabricate this modular system, which is autoclavable, biocompatible, and designed to operate following standard operating protocols (SOPs) of cellular biology. Integral to the platform is a flexible porous membrane, which is used as a cell culture substrate similarly to a commercial transwell insert. Multimodal electrochemical sensors fabricated on the membrane allow direct access to cells and their products. A pair of gold electrodes on the top side of the membrane measures impedance over the course of cell attachment and growth, characterized by an exponential decrease (~160% at 10 Hz) due to an increase in the double layer capacitance from secreted extracellular matrix (ECM) proteins. Cyclic voltammetry (CV) sensor electrodes, fabricated on the bottom side of the membrane, enable sensing of molecular release at the site of cell culture without the need for downstream fluidics. Real-time detection of ferrocene dimethanol injection across the membrane showed a three order-of-magnitude higher signal at the membrane than in the bulk media after reaching equilibrium. This modular sensor-integrated transwell system allows unprecedented direct, real-time, and noninvasive access to physical and biochemical information, which cannot be obtained in a conventional transwell system.
Keywords: Chemistry; Electrical and electronic engineering.
© The Author(s) 2020.
Conflict of interest statement
Conflict of interestThe authors declare that they do not have any conflicts of interest.
Figures



References
-
- Corning. Transwell Permeable Supports Selection and Use Guide. Corning Inc. 1–11 (2006).
-
- Marshall J. Cell migration transwell assay. Methods. 2011;769:111–136.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
