Two-year results of a randomised trial comparing 4- versus 12-weekly bone-targeted agent use in patients with bone metastases from breast or castration-resistant prostate cancer
- PMID: 34567960
- PMCID: PMC8449269
- DOI: 10.1016/j.jbo.2021.100388
Two-year results of a randomised trial comparing 4- versus 12-weekly bone-targeted agent use in patients with bone metastases from breast or castration-resistant prostate cancer
Abstract
Background: We present the 2-year results of a randomised trial comparing 4- versus 12-weekly bone-targeting agents (BTAs) in patients with bone metastases from breast or castration-resistant prostate cancer (CRPC).
Patients and methods: Patients with bone metastases from breast or CRPC, who were going to start or were already receiving BTAs, were randomised to 4- or 12-weekly BTA treatment for 2 years. The endpoints were: symptomatic skeletal events (SSE) rates, time to SSEs, toxicity and cost-effectiveness.
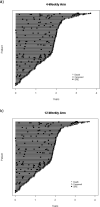
Results: Of 263 patients (160 breast cancer, 103 CRPC), 133 (50.6%) and 130 (49.4%) were randomised to the 4- and 12-weekly groups, respectively. BTAs included denosumab (56.3%), zoledronate (24.0%) and pamidronate (19.8%). After 2 years, the cumulative incidence rate (95% CI) of SSEs was 32.7% (24.6% to 41.1%) and 28.1% (20.3% to 36.4%) for the 4- and 12-weekly intervention groups respectively. The hazard ratio for time to first SSE was 0.96 (95% CI = 0.63 to 1.47). However, in a post hoc analysis, those patients who had an on-study SSE, there was a small non-statistical increased risk of subsequent SSEs among patients on the 12-weekly dosing arm (HR = 1.14; 95% CI - 0.90-1.44). BTA-related toxicity rates were similar between study arms. A cost-utility analysis showed that 12-weekly BTA is cost-effective from a public payer's perspective.
Conclusion: These results in addition to those previously reported for de-escalating zoledronate, would support that de-escalation of commonly used BTAs is a reasonable and economically valid treatment option. While not statistically significant, the increase in subsequent SSEs in the 12-weekly arm requires further exploration.
Keywords: Bisphosphonates; Breast cancer; Denosumab; Prostate cancer.
© 2021 Published by Elsevier GmbH.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Hutton B., Mazzarello S., Clemons M. Dosing Strategies of Bone-Targeting Agents. JAMA internal medicine. 2015;175(11):1864–1865. - PubMed
-
- Addison C.L., Bouganim N., Hilton J., Vandermeer L., Dent S., Amir E., Hopkins S., Kuchuk I., Segal R., Song X., Gertler S., Mazzarello S., Dranitsaris G., Ooi D., Pond G., Clemons M. A phase II, multicentre trial evaluating the efficacy of de-escalated bisphosphonate therapy in metastatic breast cancer patients at low-risk of skeletal-related events. Breast Cancer Res. Treat. 2014;144(3):615–624. - PMC - PubMed
-
- Amadori D., Aglietta M., Alessi B., Gianni L., Ibrahim T., Farina G., Gaion F., Bertoldo F., Santini D., Rondena R., Bogani P., Ripamonti C.I. Efficacy and safety of 12-weekly versus 4-weekly zoledronic acid for prolonged treatment of patients with bone metastases from breast cancer (ZOOM): a phase 3, open-label, randomised, non-inferiority trial. Lancet Oncol. 2013;14(7):663–670. - PubMed
-
- Amir E., Freedman O., Carlsson L., Dranitsaris G., Tomlinson G., Laupacis A., Tannock I.F., Clemons M. Randomized feasibility study of de-escalated (every 12 wk) versus standard (every 3 to 4 wk) intravenous pamidronate in women with low-risk bone metastases from breast cancer. Am. J. Clin. Oncol. 2013;36(5):436–442. - PubMed
LinkOut - more resources
Full Text Sources

