COVID-19 Pandemic and Vaccines Update on Challenges and Resolutions
- PMID: 34568087
- PMCID: PMC8461057
- DOI: 10.3389/fcimb.2021.690621
COVID-19 Pandemic and Vaccines Update on Challenges and Resolutions
Abstract
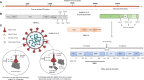
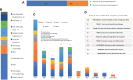
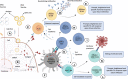
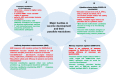
The coronavirus disease (COVID-19) is caused by a positive-stranded RNA virus called severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), belonging to the Coronaviridae family. This virus originated in Wuhan City, China, and became the cause of a multiwave pandemic that has killed 3.46 million people worldwide as of May 22, 2021. The havoc intensified with the emergence of SARS-CoV-2 variants (B.1.1.7; Alpha, B.1.351; Beta, P.1; Gamma, B.1.617; Delta, B.1.617.2; Delta-plus, B.1.525; Eta, and B.1.429; Epsilon etc.) due to mutations generated during replication. More variants may emerge to cause additional pandemic waves. The most promising approach for combating viruses and their emerging variants lies in prophylactic vaccines. Several vaccine candidates are being developed using various platforms, including nucleic acids, live attenuated virus, inactivated virus, viral vectors, and protein-based subunit vaccines. In this unprecedented time, 12 vaccines against SARS-CoV-2 have been phased in following WHO approval, 184 are in the preclinical stage, and 100 are in the clinical development process. Many of them are directed to elicit neutralizing antibodies against the viral spike protein (S) to inhibit viral entry through the ACE-2 receptor of host cells. Inactivated vaccines, to the contrary, provide a wide range of viral antigens for immune activation. Being an intracellular pathogen, the cytotoxic CD8+ T Cell (CTL) response remains crucial for all viruses, including SARS-CoV-2, and needs to be explored in detail. In this review, we try to describe and compare approved vaccines against SARS-CoV-2 that are currently being distributed either after phase III clinical trials or for emergency use. We discuss immune responses induced by various candidate vaccine formulations; their benefits, potential limitations, and effectiveness against variants; future challenges, such as antibody-dependent enhancement (ADE); and vaccine safety issues and their possible resolutions. Most of the current vaccines developed against SARS-CoV-2 are showing either promising or compromised efficacy against new variants. Multiple antigen-based vaccines (multivariant vaccines) should be developed on different platforms to tackle future variants. Alternatively, recombinant BCG, containing SARS-CoV-2 multiple antigens, as a live attenuated vaccine should be explored for long-term protection. Irrespective of their efficacy, all vaccines are efficient in providing protection from disease severity. We must insist on vaccine compliance for all age groups and work on vaccine hesitancy globally to achieve herd immunity and, eventually, to curb this pandemic.
Keywords: COVID-19; SARS CoV-2 variant; booster dose; immune response; multivariant vaccines; mutations; spike protein; vaccine.
Copyright © 2021 Khan, Hashmi, Goel, Ahmad, Gupta, Khan, Alam, Ahmed and Ansari.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

