S100B Inhibition Attenuates Intestinal Damage and Diarrhea Severity During Clostridioides difficile Infection by Modulating Inflammatory Response
- PMID: 34568098
- PMCID: PMC8461106
- DOI: 10.3389/fcimb.2021.739874
S100B Inhibition Attenuates Intestinal Damage and Diarrhea Severity During Clostridioides difficile Infection by Modulating Inflammatory Response
Abstract
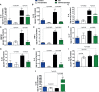
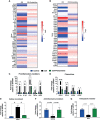
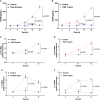
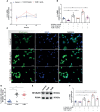
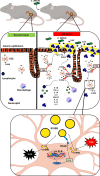
The involvement of the enteric nervous system, which is a source of S100B, in Clostridioides difficile (C. difficile) infection (CDI) is poorly understood although intestinal motility dysfunctions are known to occur following infection. Here, we investigated the role of S100B in CDI and examined the S100B signaling pathways activated in C. difficile toxin A (TcdA)- and B (TcdB)-induced enteric glial cell (EGC) inflammatory response. The expression of S100B was measured in colon tissues and fecal samples of patients with and without CDI, as well as in colon tissues from C. difficile-infected mice. To investigate the role of S100B signaling in IL-6 expression induced by TcdA and TcdB, rat EGCs were used. Increased S100B was found in colonic biopsies from patients with CDI and colon tissues from C. difficile-infected mice. Patients with CDI-promoted diarrhea exhibited higher levels of fecal S100B compared to non-CDI cases. Inhibition of S100B by pentamidine reduced the synthesis of IL-1β, IL-18, IL-6, GMCSF, TNF-α, IL-17, IL-23, and IL-2 and downregulated a variety of NFκB-related genes, increased the transcription (SOCS2 and Bcl-2) of protective mediators, reduced neutrophil recruitment, and ameliorated intestinal damage and diarrhea severity in mice. In EGCs, TcdA and TcdB upregulated S100B-mediated IL-6 expression via activation of RAGE/PI3K/NFκB. Thus, CDI appears to upregulate colonic S100B signaling in EGCs, which in turn augment inflammatory response. Inhibition of S100B activity attenuates the intestinal injury and diarrhea caused by C. difficile toxins. Our findings provide new insight into the role of S100B in CDI pathogenesis and opens novel avenues for therapeutic interventions.
Keywords: Clostridioides difficile; S100B; diarrhea; enteric glia; inflammation.
Copyright © 2021 Costa, Moura-Neto, Bolick, Guerrant, Fawad, Shin, Medeiros, Ledwaba, Kolling, Martins, Venkataraman, Warren and Brito.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Body-Malapel M., Djouina M., Waxin C., Langlois A., Gower-Rousseau C., Zerbib P., et al. . (2019). The RAGE Signaling Pathway Is Involved in Intestinal Inflammation and Represents a Promising Therapeutic Target for Inflammatory Bowel Diseases. Mucosal Immunol. 12, 468–478. doi: 10.1038/s41385-018-0119-z - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

