Reconstruction of the Swine Pulmonary Artery Using a Graft Engineered With Syngeneic Cardiac Pericytes
- PMID: 34568300
- PMCID: PMC8459923
- DOI: 10.3389/fbioe.2021.715717
Reconstruction of the Swine Pulmonary Artery Using a Graft Engineered With Syngeneic Cardiac Pericytes
Abstract
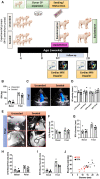
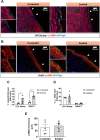
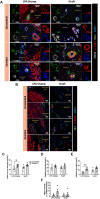
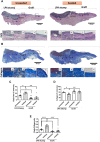
The neonatal heart represents an attractive source of regenerative cells. Here, we report the results of a randomized, controlled, investigator-blinded preclinical study, which assessed the safety and effectiveness of a matrix graft cellularized with cardiac pericytes (CPs) in a piglet model of pulmonary artery (PA) reconstruction. Within each of five trios formed by 4-week-old female littermate piglets, one element (the donor) was sacrificed to provide a source of CPs, while the other two elements (the graft recipients) were allowed to reach the age of 10 weeks. During this time interval, culture-expanded donor CPs were seeded onto swine small intestinal submucosa (SIS) grafts, which were then shaped into conduits and conditioned in a flow bioreactor. Control unseeded SIS conduits were subjected to the same procedure. Then, recipient piglets were randomized to surgical reconstruction of the left PA (LPA) with unseeded or CP-seeded SIS conduits. Doppler echocardiography and cardiac magnetic resonance imaging (CMRI) were performed at baseline and 4-months post-implantation. Vascular explants were examined using histology and immunohistochemistry. All animals completed the scheduled follow-up. No group difference was observed in baseline imaging data. The final Doppler assessment showed that the LPA's blood flow velocity was similar in the treatment groups. CMRI revealed a mismatch in the average growth of the grafted LPA and contralateral branch in both treatment groups. Histology of explanted arteries demonstrated that the CP-seeded grafts had a thicker luminal cell layer, more intraparietal arterioles, and a higher expression of endothelial nitric oxide synthase (eNOS) compared with unseeded grafts. Moreover, the LPA stump adjacent to the seeded graft contained more elastin and less collagen than the unseeded control. Syngeneic CP engineering did not accomplish the primary goal of supporting the graft's growth but was able to improve secondary outcomes, such as the luminal cellularization and intraparietal vascularization of the graft, and elastic remodeling of the recipient artery. The beneficial properties of neonatal CPs may be considered in future bioengineering applications aiming to reproduce the cellular composition of native arteries.
Keywords: congenital heart disease; grafts; pericytes; pulmonary artery; tissue engineering.
Copyright © 2021 Alvino, Thomas, Ghorbel, Rapetto, Narayan, Kilcooley, Iacobazzi, Carrabba, Fagnano, Cathery, Avolio, Caputo and Madeddu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Albertario A., Swim M. M., Ahmed E. M., Iacobazzi D., Yeong M., Madeddu P., et al. (2019). Successful Reconstruction of the Right Ventricular Outflow Tract by Implantation of Thymus Stem Cell Engineered Graft in Growing Swine. JACC: Basic Transl. Sci. 4, 364–384. 10.1016/j.jacbts.2019.02.001 - DOI - PMC - PubMed
-
- Avolio E., Rodriguez-Arabaolaza I., Spencer H. L., Riu F., Mangialardi G., Slater S. C., et al. (2015). Expansion and Characterization of Neonatal Cardiac Pericytes Provides a Novel Cellular Option for Tissue Engineering in Congenital Heart Disease. J. Am. Heart Assoc. 4, e002043. 10.1161/JAHA.115.002043 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

