Network Pharmacology and Experimental Validation Reveal the Effects of Chidamide Combined With Aspirin on Acute Myeloid Leukemia-Myelodysplastic Syndrome Cells Through PI3K/AKT Pathway
- PMID: 34568314
- PMCID: PMC8458633
- DOI: 10.3389/fcell.2021.685954
Network Pharmacology and Experimental Validation Reveal the Effects of Chidamide Combined With Aspirin on Acute Myeloid Leukemia-Myelodysplastic Syndrome Cells Through PI3K/AKT Pathway
Abstract
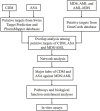
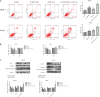
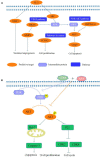
Chidamide (CDM), a novel histone deacetylase inhibitor, is currently used for patients with peripheral T-cell lymphoma. Aspirin (ASA), an anti-inflammatory drug, has been shown to exert anticancer activity. Herein, we investigated the effect of CDM combined with ASA on myelodysplastic syndromes-derived acute myeloid leukemia (AML-MDS) cells and explored the underlying mechanism. The putative targets of CDM and ASA were predicted by network pharmacology approach. GO functional and KEGG pathway enrichment analyses were performed by DAVID. Furthermore, experimental validation was conducted by Cell Counting Kit-8 assay, Flow cytometry and Western blotting. Network pharmacology analysis revealed 36 AML-MDS-related overlapping genes that were targets of CDM and ASA, while 10 hub genes were identified by the plug-in cytoHubba in Cytoscape. Pathway enrichment analysis indicated CDM and ASA significantly affected PI3K/AKT signaling pathway. Functional experiments demonstrated that the combination of CDM and ASA had a remarkable synergistic anti-proliferative effect by blocking the cell cycle in G0/G1 phase and inducing apoptosis. Mechanistically, the combination treatment significantly down-regulated the phosphorylation levels of PI3K and AKT. In addition, insulin-like growth factor 1 (IGF-1), an activator of PI3K/AKT pathway, reversed the effects of the combination treatment. Our findings suggested that CDM combined with ASA exerted a synergetic inhibitory effect on cell growth by inactivating PI3K/AKT pathway, which might pave the way for effective treatments of AML-MDS.
Keywords: PI3K/Akt pathway; aspirin; chidamide; myelodysplastic syndromes; network pharmacology.
Copyright © 2021 Liang, Zhou, Cai, Rodrigues-Lima, Chi and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Chidamide Inhibits Cell Proliferation via the PI3K/AKT Pathway in K562 Cells Based on Network Pharmacology and Experimental Validation.Curr Pharm Des. 2021;27(26):2990-2998. doi: 10.2174/1381612827666210701152250. Curr Pharm Des. 2021. PMID: 34218775
-
Chidamide, a novel histone deacetylase inhibitor, inhibits the viability of MDS and AML cells by suppressing JAK2/STAT3 signaling.Am J Transl Res. 2016 Jul 15;8(7):3169-78. eCollection 2016. Am J Transl Res. 2016. PMID: 27508038 Free PMC article.
-
Effects of chidamide and its combination with decitabine on proliferation and apoptosis of leukemia cell lines.Am J Transl Res. 2018 Aug 15;10(8):2567-2578. eCollection 2018. Am J Transl Res. 2018. PMID: 30210693 Free PMC article.
-
Phosphoinositide 3-kinase/Akt signaling pathway and its therapeutical implications for human acute myeloid leukemia.Leukemia. 2006 Jun;20(6):911-28. doi: 10.1038/sj.leu.2404245. Leukemia. 2006. PMID: 16642045 Review.
-
Targeting the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin module for acute myelogenous leukemia therapy: from bench to bedside.Curr Med Chem. 2007;14(19):2009-23. doi: 10.2174/092986707781368423. Curr Med Chem. 2007. PMID: 17691943 Review.
Cited by
-
Obesity and Leukemia: Biological Mechanisms, Perspectives, and Challenges.Curr Obes Rep. 2024 Mar;13(1):1-34. doi: 10.1007/s13679-023-00542-z. Epub 2023 Dec 30. Curr Obes Rep. 2024. PMID: 38159164 Free PMC article. Review.
-
MEK/ERK and PI3K/AKT pathway inhibitors affect the transformation of myelodysplastic syndrome into acute myeloid leukemia via H3K27me3 methylases and de‑methylases.Int J Oncol. 2023 Dec;63(6):140. doi: 10.3892/ijo.2023.5588. Epub 2023 Nov 3. Int J Oncol. 2023. PMID: 37921060 Free PMC article.
-
Exploration of the mechanism of fraxetin in treating acute myeloid leukemia based on network pharmacology and experimental verification.Heliyon. 2024 Jul 16;10(15):e34717. doi: 10.1016/j.heliyon.2024.e34717. eCollection 2024 Aug 15. Heliyon. 2024. PMID: 39166080 Free PMC article.
-
Decoding the Mechanism of Shen Qi Sha Bai Decoction in Treating Acute Myeloid Leukemia Based on Network Pharmacology and Molecular Docking.Front Cell Dev Biol. 2021 Dec 20;9:796757. doi: 10.3389/fcell.2021.796757. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34988084 Free PMC article.
-
Advances in the role of the IGF signaling system in myelodysplastic syndromes and acute myeloid leukemia.Front Oncol. 2025 Jun 24;15:1540426. doi: 10.3389/fonc.2025.1540426. eCollection 2025. Front Oncol. 2025. PMID: 40630213 Free PMC article. Review.
References
-
- Banerjee K., Das S., Sarkar A., Chatterjee M., Biswas J., Choudhuri S. K. (2016). A copper chelate induces apoptosis and overcomes multidrug resistance in T-cell acute lymphoblastic leukemia through redox imbalance and inhibition of EGFR/PI3K/Akt expression. Biomed. Pharmacother. 84 71–92. 10.1016/j.biopha.2016.08.056 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

