WW, PH and C-Terminal Domains Cooperate to Direct the Subcellular Localizations of PLEKHA5, PLEKHA6 and PLEKHA7
- PMID: 34568338
- PMCID: PMC8458771
- DOI: 10.3389/fcell.2021.729444
WW, PH and C-Terminal Domains Cooperate to Direct the Subcellular Localizations of PLEKHA5, PLEKHA6 and PLEKHA7
Abstract
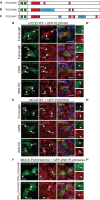
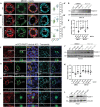
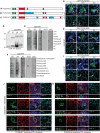
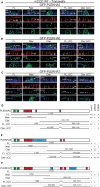
PLEKHA5, PLEKHA6, and PLEKHA7 (WW-PLEKHAs) are members of the PLEKHA family of proteins that interact with PDZD11 through their tandem WW domains. WW-PLEKHAs contribute to the trafficking and retention of transmembrane proteins, including nectins, Tspan33, and the copper pump ATP7A, at cell-cell junctions and lateral membranes. However, the structural basis for the distinct subcellular localizations of PLEKHA5, PLEKHA6, and PLEKHA7 is not clear. Here we expressed mutant and chimeric proteins of WW-PLEKHAs in cultured cells to clarify the role of their structural domains in their localization. We found that the WW-mediated interaction between PLEKHA5 and PDZD11 is required for their respective association with cytoplasmic microtubules. The PH domain of PLEKHA5 is required for its localization along the lateral plasma membrane and promotes the lateral localization of PLEKHA7 in a chimeric molecule. Although the PH domain of PLEKHA7 is not required for its localization at the adherens junctions (AJ), it promotes a AJ localization of chimeric proteins. The C-terminal region of PLEKHA6 and PLEKHA7 and the coiled-coil region of PLEKHA7 promote their localization at AJ of epithelial cells. These observations indicate that the localizations of WW-PLEKHAs at specific subcellular sites, where they recruit PDZD11, are the result of multiple cooperative protein-lipid and protein-protein interactions and provide a rational basis for the identification of additional proteins involved in trafficking and sorting of WW-PLEKHAs.
Keywords: PDZD11; PLEKHA5; PLEKHA6; PLEKHA7; WW domain; pleckstrin homology domain-containing family A.
Copyright © 2021 Sluysmans, Méan, Jond and Citi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Barbitoff Y. A., Serebryakova E. A., Nasykhova Y. A., Predeus A. V., Polev D. E., Shuvalova A. R., et al. (2018). Identification of novel candidate markers of type 2 diabetes and obesity in russia by exome sequencing with a limited sample size. Genes (Basel) 9:415. 10.3390/genes9080415 - DOI - PMC - PubMed
-
- Godi A., Di Campli A., Konstantakopoulos A., Di Tullio G., Alessi D. R., Kular G. S., et al. (2004). FAPPs control Golgi-to-cell-surface membrane traffic by binding to ARF and PtdIns(4)P. Nat. Cell. Biol. 6 393–404. - PubMed
LinkOut - more resources
Full Text Sources

