Preparation of pH-Responsive Vesicular Deferasirox: Evidence from In Silico, In Vitro, and In Vivo Evaluations
- PMID: 34568700
- PMCID: PMC8459436
- DOI: 10.1021/acsomega.1c03816
Preparation of pH-Responsive Vesicular Deferasirox: Evidence from In Silico, In Vitro, and In Vivo Evaluations
Abstract
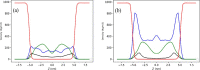
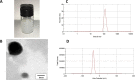
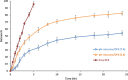
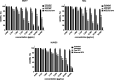
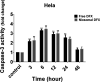
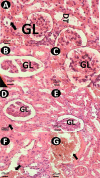
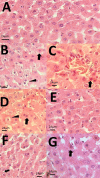
pH-sensitive nanocarriers can effectively deliver anticancer drugs to tumors and reduce the adverse effects of conventional chemotherapy. In this light, we prepared a novel pH-responsive deferasirox (DFX)-loaded vesicle and comprehensively performed in silico, in vitro, and in vivo studies to examine the properties of the newly synthesized formulation. Physiochemical assessment of the developed formulations showed that they have an average size (107 ± 2 nm), negative zeta potential (-29.1 ± 1.5 mV), high encapsulation efficiency (84.2 ± 2.6%), and a pH-responsive release. Using the molecular dynamics simulation, the structural and dynamic properties of ergosterol-containing niosomes (ST60/Ergo) in the presence of DFX molecules were analyzed and showed a good interaction between DFX and vesicle components. Cytotoxic assessment showed that niosomal DFX exhibited a greater cytotoxic effect than free DFX in both human cancer cells (MCF-breast cancer and Hela cervical cancer) and induced evident morphological features of apoptotic cell death. No marked difference between the ability of free and niosomal DFX was found in activating caspase-3 in Hela cells. Eight weeks of intraperitoneal administrations of free DFX at three doses caused a significant increase in serum biochemical parameters and liver lipid peroxidation. Treatment with 5 mg/kg dose of niosomal DFX caused a significant increase in serum creatinine (P < 0.05); however, other parameters remained unchanged. On the other hand, administration of niosomal DFX at the highest dose (10 mg/kg) significantly increased serum creatinine (P < 0.05), BUN, and serum liver enzymes compared to the control rats (P < 0.001). Based on the results, the application of pH-responsive DFX-loaded niosomes, as a novel drug delivery platform, may yield promising results in cancer treatment.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Rahdar A.; Hajinezhad M. R.; Sargazi S.; Barani M.; Bilal M.; Kyzas G. Z. Deferasirox-loaded pluronic nanomicelles: Synthesis, characterization, in vitro and in vivo studies. J. Mol. Liq. 2021, 323, 114605.10.1016/j.molliq.2020.114605. - DOI
-
- Taghavi F.; Saljooghi A. S.; Gholizadeh M.; Ramezani M. Deferasirox-coated iron oxide nanoparticles as a potential cytotoxic agent. MedChemComm 2016, 7, 2290–2298. 10.1039/c6md00293e. - DOI
-
- Taghavi F.; Gholizadeh M.; Saljooghi A. S. Deferasirox loaded on fumed silica nanoparticles used in cancer treatment. New J. Chem. 2016, 40, 2696–2703. 10.1039/c5nj02790j. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

