Tissue Engineering the Pinna: Comparison and Characterization of Human Decellularized Auricular Biological Scaffolds
- PMID: 34568774
- PMCID: PMC8456428
- DOI: 10.1021/acsabm.1c00766
Tissue Engineering the Pinna: Comparison and Characterization of Human Decellularized Auricular Biological Scaffolds
Abstract
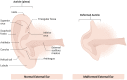
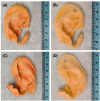
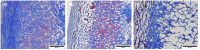
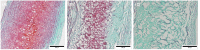
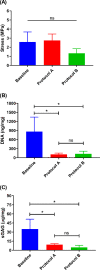
Decellularization is one of the promising techniques in tissue engineering used to create a biological scaffold for subsequent repopulation with the patient's own cells. This study aims to compare two different decellularization protocols to optimize the process of auricle decellularization by assessing and characterizing the decellularization effects on human auricular cartilage. Herein, 12 pairs (8 females, 4 males) of freshly frozen adult human cadaveric auricles were de-epithelialized and defatted leaving only the cartilaginous framework. An auricle from each pair was randomly assigned to either protocol A (latrunculin B-based decellularization) or protocol B (trypsin-based decellularization). Gross examination of the generated scaffolds demonstrated preservation of the auricles' contours and a change in color from pinkish-white to yellowish-white. Hematoxylin and eosin staining demonstrated empty cartilaginous lacunae in both study groups, which confirms the depletion of cells. However, there was greater preservation of the extracellular matrix in auricles decellularized with protocol A as compared to protocol B. Comparing protocol A to protocol B, Masson's trichrome and Safranin-O stains also demonstrated noticeable preservation of collagen and proteoglycans, respectively. Additionally, scanning electron micrographs demonstrated preservation of the cartilaginous microtopography in both study groups. Biomechanical testing demonstrated a substantial decrease in Young's modulus after decellularization using protocol B (1.3 MPa), albeit not significant (P-value > 0.05) when compared to Young's modulus prior to decellularization (2.6 MPa) or after decellularization with protocol A (2.7 MPa). A DNA quantification assay demonstrated a significant drop (P-value < 0.05) in the DNA content after decellularization with protocol A (111.0 ng/mg) and protocol B (127.6 ng/mg) in comparison to before decellularization (865.3 ng/mg). Overall, this study demonstrated effective decellularization of human auricular cartilage, and it is concluded that protocol A provided greater preservation of the extracellular matrix and biomechanical characteristics. These findings warrant proceeding with the assessment of inflammation and cell migration in a decellularized scaffold using an animal model.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

