Hepatocyte activity of the cholesterol sensor smoothened regulates cholesterol and bile acid homeostasis in mice
- PMID: 34568800
- PMCID: PMC8449244
- DOI: 10.1016/j.isci.2021.103089
Hepatocyte activity of the cholesterol sensor smoothened regulates cholesterol and bile acid homeostasis in mice
Abstract
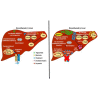
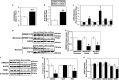
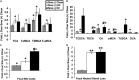
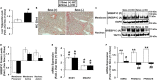
Cellular cholesterol is regulated by at least two transcriptional mechanisms involving sterol-regulatory-element-binding proteins (SREBPs) and liver X receptors (LXRs). Although SREBP and LXR pathways are the predominant mechanisms that sense cholesterol in the endoplasmic reticulum and nucleus to alter sterol-regulated gene expression, evidence suggests cholesterol in plasma membrane can be sensed by proteins in the Hedgehog (Hh) pathway which regulate organ self-renewal and are a morphogenic driver during embryonic development. Cholesterol interacts with the G-protein-coupled receptor Smoothened (Smo), which impacts downstream Hh signaling. Although evidence suggests cholesterol influences Hh signaling, it is not known whether Smo-dependent sterol sensing impacts cholesterol homeostasis in vivo. We examined dietary-cholesterol-induced reorganization of whole-body sterol and bile acid (BA) homeostasis in adult mice with inducible hepatocyte-specific Smo deletion. These studies demonstrate Smo in hepatocytes plays a regulatory role in sensing and feedback regulation of cholesterol balance driven by excess dietary cholesterol.
Keywords: Lipid; Molecular biology; Molecular physiology.
© 2021 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
GRAMD1/ASTER-mediated cholesterol transport promotes Smoothened cholesterylation at the endoplasmic reticulum.EMBO J. 2023 Feb 1;42(3):e111513. doi: 10.15252/embj.2022111513. Epub 2022 Dec 16. EMBO J. 2023. PMID: 36524353 Free PMC article.
-
Differential gene regulation of StarD4 and StarD5 cholesterol transfer proteins. Activation of StarD4 by sterol regulatory element-binding protein-2 and StarD5 by endoplasmic reticulum stress.J Biol Chem. 2005 May 13;280(19):19410-8. doi: 10.1074/jbc.M501778200. Epub 2005 Mar 10. J Biol Chem. 2005. PMID: 15760897
-
REV-ERBalpha participates in circadian SREBP signaling and bile acid homeostasis.PLoS Biol. 2009 Sep;7(9):e1000181. doi: 10.1371/journal.pbio.1000181. Epub 2009 Sep 1. PLoS Biol. 2009. PMID: 19721697 Free PMC article.
-
The interplay of Patched, Smoothened and cholesterol in Hedgehog signaling.Curr Opin Cell Biol. 2019 Dec;61:31-38. doi: 10.1016/j.ceb.2019.06.008. Epub 2019 Jul 29. Curr Opin Cell Biol. 2019. PMID: 31369952 Review.
-
Sterol regulatory element-binding protein family as global regulators of lipid synthetic genes in energy metabolism.Vitam Horm. 2002;65:167-94. doi: 10.1016/s0083-6729(02)65064-2. Vitam Horm. 2002. PMID: 12481547 Review.
Cited by
-
Hepatocyte Smoothened Activity Controls Susceptibility to Insulin Resistance and Nonalcoholic Fatty Liver Disease.Cell Mol Gastroenterol Hepatol. 2023;15(4):949-970. doi: 10.1016/j.jcmgh.2022.12.008. Epub 2022 Dec 16. Cell Mol Gastroenterol Hepatol. 2023. PMID: 36535507 Free PMC article.
-
The senescence-associated secretome of Hedgehog-deficient hepatocytes drives MASLD progression.J Clin Invest. 2024 Aug 27;134(19):e180310. doi: 10.1172/JCI180310. J Clin Invest. 2024. PMID: 39190624 Free PMC article.
-
Cryptococcosis, tuberculosis, and a kidney cancer fail to fit the atherosclerosis paradigm for foam cell lipid content.J Immunol. 2025 Jun 1;214(6):1358-1369. doi: 10.1093/jimmun/vkaf038. J Immunol. 2025. PMID: 40156376 Free PMC article.
-
UPLC-Q-TOF-MS-based unbiased serum metabolomics investigation of cholangiocarcinoma.Front Mol Biosci. 2025 Apr 7;12:1549223. doi: 10.3389/fmolb.2025.1549223. eCollection 2025. Front Mol Biosci. 2025. PMID: 40260405 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

