A combination of mRNA features influence the efficiency of leaderless mRNA translation initiation
- PMID: 34568822
- PMCID: PMC8459731
- DOI: 10.1093/nargab/lqab081
A combination of mRNA features influence the efficiency of leaderless mRNA translation initiation
Abstract
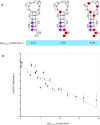
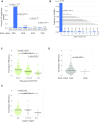
Bacterial translation is thought to initiate by base pairing of the 16S rRNA and the Shine-Dalgarno sequence in the mRNA's 5' untranslated region (UTR). However, transcriptomics has revealed that leaderless mRNAs, which completely lack any 5' UTR, are broadly distributed across bacteria and can initiate translation in the absence of the Shine-Dalgarno sequence. To investigate the mechanism of leaderless mRNA translation initiation, synthetic in vivo translation reporters were designed that systematically tested the effects of start codon accessibility, leader length, and start codon identity on leaderless mRNA translation initiation. Using these data, a simple computational model was built based on the combinatorial relationship of these mRNA features that can accurately classify leaderless mRNAs and predict the translation initiation efficiency of leaderless mRNAs. Thus, start codon accessibility, leader length, and start codon identity combine to define leaderless mRNA translation initiation in bacteria.
© The Author(s) 2021. Published by Oxford University Press on behalf of NAR Genomics and Bioinformatics.
Figures





References
-
- Kurland C.G., Ehrenberg M.. Growth-optimizing accuracy of gene expression. Annu. Rev. Biophys. Biophys. Chem. 1987; 16:291–317. - PubMed
-
- Rodnina M.V., Wintermeyer W.. Fidelity of aminoacyl-tRNA selection on the ribosome: kinetic and structural mechanisms. Annu. Rev. Biochem. 2001; 70:415–435. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

