Bortezomib induces anti-multiple myeloma immune response mediated by cGAS/STING pathway activation
- PMID: 34568832
- PMCID: PMC8462183
- DOI: 10.1158/2643-3230.BCD-21-0047
Bortezomib induces anti-multiple myeloma immune response mediated by cGAS/STING pathway activation
Abstract
Proteasome inhibitor bortezomib induces apoptosis in multiple myeloma (MM) cells, and has transformed patient outcome. Using in vitro as well as in vivo immunodeficient and immunocompetent murine MM models, we here show that bortezomib also triggers immunogenic cell death (ICD) characterized by exposure of calreticulin on dying MM cells, phagocytosis of tumor cells by dendritic cells, and induction of MM specific immunity. We identify a bortezomib-triggered specific ICD-gene signature associated with better outcome in two independent MM patient cohorts. Importantly, bortezomib stimulates MM cells immunogenicity via activation of cGAS/STING pathway and production of type-I interferons; and STING agonists significantly potentiate bortezomib-induced ICD. Our studies therefore delineate mechanisms whereby bortezomib exerts immunotherapeutic activity, and provide the framework for clinical trials of STING agonists with bortezomib to induce potent tumor-specific immunity and improve patient outcome in MM.
Keywords: STING; bortezomib; immunogenic cell death (ICD); immunotherapy; multiple myeloma.
Conflict of interest statement
Conflict of interest KCA serves on advisory boards to Janssen, Pfizer, Astrazeneca, Amgen, Precision Biosciences, Mana, Starton, and Raqia, and is a Scientific Founder of OncoPep and C4 Therapeutics. NCM serves on advisory boards/consultant to Takeda, BMS, Celgene, Janssen, Amgen, AbbVie, Oncopep, Karyopharm, Adaptive Biotechnology, and Novartis and is a Scientific Founder of Oncopep. PGR serves on advisory boards for Celgene/BMS, Janssen, Jazz Pharmaceuticals, Karyopharm Therapeutics, Oncopeptides, Sanofi, Takeda and Secura Bio. The other authors have no other relevant conflict of interest.
Figures
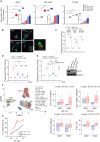




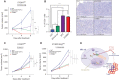
Comment in
-
Bortezomib Induces Immunogenic Cell Death in Multiple Myeloma.Blood Cancer Discov. 2021 May 26;2(5):405-407. doi: 10.1158/2643-3230.BCD-21-0059. eCollection 2021 Sep. Blood Cancer Discov. 2021. PMID: 34661160 Free PMC article.
References
-
- Kumar SK, Rajkumar V, Kyle RA, van Duin M, Sonneveld P, Mateos MV, et al. Multiple myeloma. Nat Rev Dis Primers 2017;3:17046. - PubMed
-
- Nakamura K, Smyth MJ, Martinet L.Cancer immunoediting and immune dysregulation in multiple myeloma. Blood 2020;136:2731–40. - PubMed
-
- Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G.Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell 2015;28:690–714. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

