Met is required for oligodendrocyte progenitor cell migration in Danio rerio
- PMID: 34568921
- PMCID: PMC8473979
- DOI: 10.1093/g3journal/jkab265
Met is required for oligodendrocyte progenitor cell migration in Danio rerio
Abstract
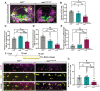
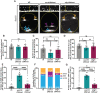
During vertebrate central nervous system development, most oligodendrocyte progenitor cells (OPCs) are specified in the ventral spinal cord and must migrate throughout the neural tube until they become evenly distributed, occupying non-overlapping domains. While this process of developmental OPC migration is well characterized, the nature of the molecular mediators that govern it remain largely unknown. Here, using zebrafish as a model, we demonstrate that Met signaling is required for initial developmental migration of OPCs, and, using cell-specific knock-down of Met signaling, show that Met acts cell-autonomously in OPCs. Taken together, these findings demonstrate in vivo, the role of Met signaling in OPC migration and provide new insight into how OPC migration is regulated during development.
Keywords: Met receptor; glial migration; oligodendrocyte progenitor cells; spinal cord development; zebrafish.
© The Author(s) 2021. Published by Oxford University Press on behalf of Genetics Society of America.
Figures





References
-
- Auer F, Vagionitis S, Czopka T.. 2018. Evidence for myelin sheath remodeling in the CNS revealed by in vivo imaging. Curr Biol. 28:549–559.e3. doi:10.1016/j.cub.2018.01.017. - PubMed
-
- Bardelli A, Longati P, Williams TA, Benvenuti S, Comoglio PM.. 1999. A peptide representing the carboxyl-terminal tail of the Met receptor inhibits kinase activity and invasive growth. J Biol Chem. 274:29274–29281. doi:10.1074/jbc.274.41.29274. - PubMed
-
- Birchmeier C, Gherardi E.. 1998. Developmental roles of HGF/SF and its receptor, the c-Met tyrosine kinase. Trends Cell Biol. 8:404–410. doi:10.1016/S0962-8924(98)01359-2. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
