Development of antifibrotic therapy for stricturing Crohn's disease: lessons from randomized trials in other fibrotic diseases
- PMID: 34569264
- PMCID: PMC8742742
- DOI: 10.1152/physrev.00005.2021
Development of antifibrotic therapy for stricturing Crohn's disease: lessons from randomized trials in other fibrotic diseases
Abstract
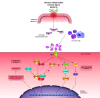
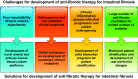
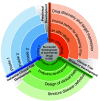
Intestinal fibrosis is considered an inevitable complication of Crohn's disease (CD) that results in symptoms of obstruction and stricture formation. Endoscopic or surgical treatment is required to treat the majority of patients. Progress in the management of stricturing CD is hampered by the lack of effective antifibrotic therapy; however, this situation is likely to change because of recent advances in other fibrotic diseases of the lung, liver, and skin. In this review, we summarize data from randomized controlled trials (RCTs) of antifibrotic therapies in these conditions. Multiple compounds have been tested for antifibrotic effects in other organs. According to their mechanisms, they were categorized into growth factor modulators, inflammation modulators, 5-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, intracellular enzymes and kinases, renin-angiotensin system (RAS) modulators, and others. From our review of the results from the clinical trials and discussion of their implications in the gastrointestinal tract, we have identified several molecular candidates that could serve as potential therapies for intestinal fibrosis in CD.
Keywords: Crohn’s disease; clinical trials; end point; stricture.
Conflict of interest statement
D.B. is on the advisory board of or is a consultant for AbbVie, Amgen, Arena, BNG Service, Celltrion, Dr. Falk Foundation, Ferring, Galapagos Janssen-Cilag, Medical Tribune, MSD, Pfizer, Pharmacosmos, Roche, Takeda, Thieme, Tillotts Pharma, and Vifor. V.J. has received consulting fees from AbbVie, Eli Lilly, GlaxoSmithKline, Arena Pharmaceuticals, Genetech, Pendopharm, Sandoz, Merck, Takeda, Janssen, Alimentiv Inc., Topivert, and Celltrion and speaker’s fees from Takeda, Janssen, Shire, Ferring, Abbvie, and Pfizer. B.G.F. has received grant/research support from AbbVie Inc., Amgen Inc., AstraZeneca/MedImmune Ltd., Atlantic Pharmaceuticals Ltd., Boehringer-Ingelheim, Celgene Corporation, Celltech, Genentech Inc/Hoffmann-La Roche Ltd., Gilead Sciences Inc., GlaxoSmithKline (GSK), Janssen Research & Development LLC., Pfizer Inc., Receptos Inc./Celgene International, Sanofi, Santarus Inc., Takeda Development Center Americas Inc., Tillotts Pharma AG, and UCB, consulting fees from Abbott/AbbVie, Akebia Therapeutics, Allergan, Amgen, Applied Molecular Transport Inc., Aptevo Therapeutics, Astra Zeneca, Atlantic Pharma, Avir Pharma, Biogen Idec, BioMx Israel, Boehringer-Ingelheim, Bristol-Myers Squibb, Calypso Biotech, Celgene, Elan/Biogen, EnGene, Ferring Pharma, Roche/Genentech, Galapagos, GiCare Pharma, Gilead, Gossamer Pharma, GSK, Inception IBD Inc, JnJ/Janssen, Kyowa Kakko Kirin Co Ltd., Lexicon, Lilly, Lycera BioTech, Merck, Mesoblast Pharma, Millennium, Nestle, Nextbiotix, Novonordisk, Pfizer, Prometheus Therapeutics and Diagnostics, Progenity, Protagonist, Receptos, Salix Pharma, Shire, Sienna Biologics, Sigmoid Pharma, Sterna Biologicals, Synergy Pharma Inc., Takeda, Teva Pharma, TiGenix, Tillotts, UCB Pharma, Vertex Pharma, Vivelix Pharma, VHsquared Ltd., and Zyngenia, and speaker’s bureau fees from Abbott/AbbVie, JnJ/Janssen, Lilly, Takeda, Tillotts, and UCB Pharma; is a scientific advisory board member for Abbott/AbbVie, Allergan, Amgen, Astra Zeneca, Atlantic Pharma, Avaxia Biologics Inc., Boehringer-Ingelheim, Bristol-Myers Squibb, Celgene, Centocor Inc., Elan/Biogen, Galapagos, Genentech/Roche, JnJ/Janssen, Merck, Nestle, Novartis, Novonordisk, Pfizer, Prometheus Laboratories, Protagonist, Salix Pharma, Sterna Biologicals, Takeda, Teva, TiGenix, Tillotts Pharma AG, and UCB Pharma; and is the Senior Scientific Officer of Alimentiv Inc. F.R. is a consultant to Agomab, Allergan, AbbVie, Boehringer-Ingelheim, Celgene, Cowen, Genentech, Gilead, Gossamer, Guidepoint, Helmsley, Index Pharma, Jannsen, Koutif, Metacrine, Morphic, Pfizer, Pliant, Prometheus Biosciences, Receptos, RedX, Roche, Samsung, Takeda, Techlab, Thetis, and UCB and receives funding from the Crohn’s and Colitis Foundation of America, the Helmsley Charitable Trust, Kenneth Rainin Foundation, and the National Institutes of Health. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures



References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

