A palmitoylation code controls PI4KIIIα complex formation and PI(4,5)P2 homeostasis at the plasma membrane
- PMID: 34569608
- PMCID: PMC8592054
- DOI: 10.1242/jcs.259365
A palmitoylation code controls PI4KIIIα complex formation and PI(4,5)P2 homeostasis at the plasma membrane
Abstract
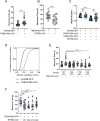
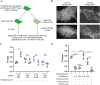
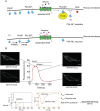
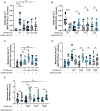
Phosphatidylinositol 4-kinase IIIα (PI4KIIIα) is the major enzyme responsible for generating phosphatidylinositol (4)-phosphate [PI(4)P] at the plasma membrane. This lipid kinase forms two multicomponent complexes, both including a palmitoylated anchor, EFR3. Whereas both PI4KIIIα complexes support production of PI(4)P, the distinct functions of each complex and mechanisms underlying the interplay between them remain unknown. Here, we present roles for differential palmitoylation patterns within a tri-cysteine motif in EFR3B (Cys5, Cys7 and Cys8) in controlling the distribution of PI4KIIIα between these two complexes at the plasma membrane and corresponding functions in phosphoinositide homeostasis. Spacing of palmitoyl groups within three doubly palmitoylated EFR3B 'lipoforms' affects both interactions between EFR3B and TMEM150A, a transmembrane protein governing formation of a PI4KIIIα complex functioning in rapid phosphatidylinositol (4,5)-bisphosphate [PI(4,5)P2] resynthesis following phospholipase C signaling, and EFR3B partitioning within liquid-ordered and -disordered regions of the plasma membrane. This work identifies a palmitoylation code involved in controlling protein-protein and protein-lipid interactions that affect a plasma membrane-resident lipid biosynthetic pathway.
Keywords: EFR3; Membrane microdomains; PI(4)P; PI4KIIIα; Palmitoylation; Phosphoinositides; TMEM150A.
© 2021. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

