Membrane protein-chimeric liposome-mediated delivery of triptolide for targeted hepatocellular carcinoma therapy
- PMID: 34569906
- PMCID: PMC8477919
- DOI: 10.1080/10717544.2021.1983072
Membrane protein-chimeric liposome-mediated delivery of triptolide for targeted hepatocellular carcinoma therapy
Abstract
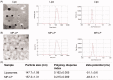
Triptolide (TPL) is a diterpenoid triepoxide with broad antitumor efficacy, while lack of mechanism of action, severe systemic toxicity, and poor water solubility of TPL limited its usage. To unveil the mechanism of action and improve the pharmaceutical properties of TPL, here we explored the molecular mechanism of TPL and then fabricated TPL-loaded membrane protein-chimeric liposomes (TPL@MP-LP) and tested its anticancer efficacy against hepatocellular carcinoma (HCC). CCK8 assay, colony formation assay, EdU assay, and flow cytometry were used to examine the activity of TPL. RNA sequence and gain-and-loss of function assays were used to explore the molecular mechanisms. TPL@MP-LP was characterized by size, zeta potential, polydispersity index, and transmission electron microscopy. Cellular uptake and cell viability assay were performed to evaluate the internalization and anticancer efficacy of TPL@MP-LP in vitro. Biodistribution and in vivo antitumor efficacy of TPL@MP-LP were evaluated on orthotopic HCC mice models. TPL robustly inhibited HCC cells by inducing cell proliferation arrest, apoptosis via the mitochondrial pathway, and necroptosis via RIPK1/RIPK3/MLKL signaling. TPL was successfully loaded into MP-LP, with a drug-loading capacity of 5.62 ± 0.80%. MP-LP facilitated TPL internalization and TPL@MP-LP exerted enhanced anticancer efficacy against Huh7 cells. TPL@MP-LP showed targeting ability to the tumor site. More importantly, TPL@MP-LP treatment suppressed tumor growth but showed minimal damage to liver and renal functions. TPL exerted anticancer effects on HCC via inducing cell proliferation arrest, apoptosis, and necroptosis, and the MP-LP might be a promising delivery strategy to improve the antitumor efficacy while mitigating toxicity of TPL for HCC therapy.
Keywords: Triptolide; apoptosis; drug delivery; hepatocellular carcinoma; membrane proteins; necroptosis.
Conflict of interest statement
The authors declare no conflict of interests.
Figures





References
-
- Cartwright T, Perkins ND, L Wilson C. (2016). NFKB1: a suppressor of inflammation, ageing and cancer. Febs J 283:1812–22. - PubMed
-
- Crommelin DJA, van Hoogevest P, Storm G. (2020). The role of liposomes in clinical nanomedicine development. What now? Now what? J Control Release 318:256–63. - PubMed
-
- Elnaggar MH, Abushouk AI, Hassan AHE, et al. (2021). Nanomedicine as a putative approach for active targeting of hepatocellular carcinoma. Semin Cancer Biol 69:91–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
