Role of Fucoxanthin towards Cadmium-induced renal impairment with the antioxidant and anti-lipid peroxide activities
- PMID: 34569908
- PMCID: PMC8806766
- DOI: 10.1080/21655979.2021.1973875
Role of Fucoxanthin towards Cadmium-induced renal impairment with the antioxidant and anti-lipid peroxide activities
Abstract
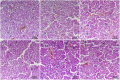
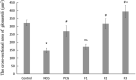
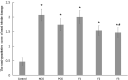
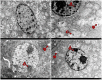
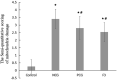
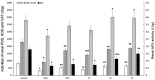
Kidney damages caused by cadmium are considered to be one of the most dangerous consequences for the human body. This study aimed to investigate the protective effects of fucoxanthin supplementation on mice models subjected to cadmium-induced kidney damage. The mice treated with cadmium chloride (CdCl2) were observed to have significantly reduced the cross-section area of glomeruli. Cadmium exposure has also caused the damage of the structural integrity of mitochondria and increased blood urea nitrogen (BUN), kidney injury molecule 1 (KIM1), and neutrophil gelatinase associated lipocalin (NGAL) levels. Peroxidase (POD), superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidase (APX) levels in cadmium-exposed mice were markedly declined. Caspase3, caspase8, and caspase9 gene expressions in association with apoptosis were dramatically elevated in renal tissues. The CdCl2 treated mice were orally administered with 50 mg/kg Shenfukang, 10 mg/kg, 25 mg/kg, and 50 mg/kg fucoxanthin for 14 days. The results revealed that high doses of fucoxanthin administration significantly decreased BUN, KIM1, NGAL levels, increasing POD, SOD, CAT, and ascorbate APX levels. Fucoxanthin administration also promoted recovery of the renal functions, micro-structural organization, and ultra-structural organization in the renal cells. In summary, the ameliorative effects of fucoxanthin supplementation against cadmium-induced kidney damage were mediated via inhibiting oxidative stress and apoptosis, promoting the recovery of structural integrity of mitochondria.
Keywords: Apoptosis; cadmium chloride; fucoxanthin; oxidative stress; renal function.
Figures

References
-
- Nawrot T, Plusquin M, Hogervorst J, et al. Environmental exposure to cadmium and risk of cancer: a prospective population-based study. Lancet Oncol. 2006;7(2):119–126. - PubMed
-
- Satarug S, Baker JR, Urbenjapol S, et al. A global perspective on cadmium pollution and toxicity in non-occupationally exposed population. Toxicol Lett. 2003;137(1–2):65–83. - PubMed
-
- Gobe GC, Johnson DW.. Distal tubular epithelial cells of the kidney: potential support for proximal tubular cell survival after renal injury. Int J Biochem Cell Biol. 2007;39(9):1551–1561. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
