Directed Signaling Cascades in Monodisperse Artificial Eukaryotic Cells
- PMID: 34570489
- PMCID: PMC8552445
- DOI: 10.1021/acsnano.1c04219
Directed Signaling Cascades in Monodisperse Artificial Eukaryotic Cells
Abstract
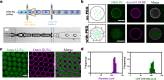
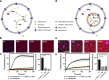
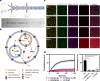
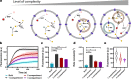
The bottom-up assembly of multicompartment artificial cells that are able to direct biochemical reactions along a specific spatial pathway remains a considerable engineering challenge. In this work, we address this with a microfluidic platform that is able to produce monodisperse multivesicular vesicles (MVVs) to serve as synthetic eukaryotic cells. Using a two-inlet polydimethylsiloxane channel design to co-encapsulate different populations of liposomes we are able to produce lipid-based MVVs in a high-throughput manner and with three separate inner compartments, each containing a different enzyme: α-glucosidase, glucose oxidase, and horseradish peroxidase. We demonstrate the ability of these MVVs to carry out directed chemical communication between the compartments via the reconstitution of size-selective membrane pores. Therefore, the signal transduction, which is triggered externally, follows a specific spatial pathway between the compartments. We use this platform to study the effects of enzyme cascade compartmentalization by direct analytical comparison between bulk, one-, two-, and three-compartment systems. This microfluidic strategy to construct complex hierarchical structures is not only suitable to study compartmentalization effects on biochemical reactions but is also applicable for developing advanced drug delivery systems as well as minimal cells in the field of bottom-up synthetic biology.
Keywords: artificial cells; bottom-up; directed signaling; enzyme cascade; microfluidics; multicompartmentalization; synthetic biology.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

