Pyronaridine-Artesunate (Pyramax) for Treatment of Artemisinin- and Piperaquine-Resistant Plasmodium falciparum in the Central Highlands of Vietnam
- PMID: 34570647
- PMCID: PMC8597783
- DOI: 10.1128/AAC.00276-21
Pyronaridine-Artesunate (Pyramax) for Treatment of Artemisinin- and Piperaquine-Resistant Plasmodium falciparum in the Central Highlands of Vietnam
Abstract
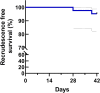
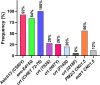
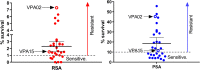
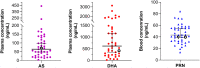
The rise in Plasmodium falciparum resistance to dihydroartemisinin-piperaquine in Vietnam justifies the need to evaluate alternative artemisinin-based combination therapies. Between July 2018 and October 2019, a single-arm trial of pyronaridine-artesunate (Pyramax, PA) was conducted in Dak Nong province, Vietnam. PA (3-day course) was administered to adults and children infected with P. falciparum. PA was well tolerated by the participants. The proportion of patients with Day 42 PCR-corrected adequate clinical and parasitological response was 95.2% (95% confidence interval [CI], 82.3 to 98.8, n = 40/42) for treating falciparum malaria. The median parasite clearance half-life was 6.7 h (range, 2.6 to 11.9) and the median parasite clearance time was 72 h (range, 12 to 132) with 44.9% (22/49) of patients having positive blood films at 72 h. The two patients that recrudesced had comparable Day 7 blood pyronaridine concentrations (39.5 and 39.0 ng/ml) to the 40 patients who did not recrudesce (median 43.4 ng/ml, 95% CI, 35.1 to 54.9). Ring-stage and piperaquine survival assays revealed that of the 29 P. falciparum isolates collected from the patients before PA treatment, 22 (75.9%) had reduced susceptibility to artemisinins and 17 (58.6%) were resistant to piperaquine. Genotyping confirmed that 92.0% (46/50) of falciparum patients were infected with parasites bearing the Pfkelch13 C580Y mutation associated with artemisinin resistance. Of these, 56.0% (28/50) of the isolates also had multiple copies of the plasmepsin 2/3 genes responsible for piperaquine resistance. Overall, PA was effective in treating P. falciparum in the Central Highlands of Vietnam. (This study has been registered at AustralianClinicalTrials.gov.au under trial ID ACTRN12618001429246.).
Keywords: Central Highlands; Pfcrt; Pfkelch13; Pfmdr1; Plasmodium falciparum; Pyramax; Vietnam; antimalarial drug resistance; dihydroartemisinin; exo-E415G; piperaquine; plasmepsin 2/3; pyronaridine; pyronaridine-artesunate.
Figures

References
-
- Thuy-Nhien N, Tuyen NK, Tong NT, Vy NT, Thanh NV, Van HT, Huong-Thu P, Quang HH, Boni MF, Dolecek C, Farrar J, Thwaites GE, Miotto O, White NJ, Hien TT. 2017. K13 Propeller Mutations in Plasmodium falciparum populations in regions of malaria endemicity in Vietnam from 2009 to 2016. Antimicrob Agents Chemother 61. 10.1128/AAC.01578-16. - DOI - PMC - PubMed
-
- Thanh NV, Thuy-Nhien N, Tuyen NT, Tong NT, Nha-Ca NT, Dong LT, Quang HH, Farrar J, Thwaites G, White NJ, Wolbers M, Hien TT. 2017. Rapid decline in the susceptibility of Plasmodium falciparum to dihydroartemisinin-piperaquine in the south of Vietnam. Malar J 16:27. 10.1186/s12936-017-1680-8. - DOI - PMC - PubMed
-
- van der Pluijm RW, Imwong M, Chau NH, Hoa NT, Thuy-Nhien NT, Thanh NV, Jittamala P, Hanboonkunupakarn B, Chutasmit K, Saelow C, Runjarern R, Kaewmok W, Tripura R, Peto TJ, Yok S, Suon S, Sreng S, Mao S, Oun S, Yen S, Amaratunga C, Lek D, Huy R, Dhorda M, Chotivanich K, Ashley EA, Mukaka M, Waithira N, Cheah PY, Maude RJ, Amato R, Pearson RD, Gonçalves S, Jacob CG, Hamilton WL, Fairhurst RM, Tarning J, Winterberg M, Kwiatkowski DP, Pukrittayakamee S, Hien TT, Day NP, Miotto O, White NJ, Dondorp AM. 2019. Determinants of dihydroartemisinin-piperaquine treatment failure in Plasmodium falciparum malaria in Cambodia, Thailand, and Vietnam: a prospective clinical, pharmacological, and genetic study. Lancet Infect Dis 19:952–961. 10.1016/S1473-3099(19)30391-3. - DOI - PMC - PubMed
-
- (WANECAM) TWANfCToAD. 2018. Pyronaridine-artesunate or dihydroartemisinin-piperaquine versus current first-line therapies for repeated treatment of uncomplicated malaria: a randomised, multicentre, open-label, longitudinal, controlled, phase 3b/4 trial. Lancet 391:1378–1390. 10.1016/S0140-6736(18)30291-5. - DOI - PMC - PubMed
-
- Rueangweerayut R, Phyo AP, Uthaisin C, Poravuth Y, Binh TQ, Tinto H, Pénali LK, Valecha N, Tien NT, Abdulla S, Borghini-Fuhrer I, Duparc S, Shin CS, Fleckenstein L. 2012. Pyronaridine-artesunate versus mefloquine plus artesunate for malaria. N Engl J Med 366:1298–1309. 10.1056/NEJMoa1007125. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

