Effectiveness of BNT162b2 Vaccine in Adolescents during Outbreak of SARS-CoV-2 Delta Variant Infection, Israel, 2021
- PMID: 34570694
- PMCID: PMC8544958
- DOI: 10.3201/eid2711.211886
Effectiveness of BNT162b2 Vaccine in Adolescents during Outbreak of SARS-CoV-2 Delta Variant Infection, Israel, 2021
Abstract
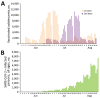
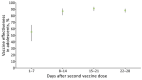
In Israel, the BNT162b2 vaccine against severe acute respiratory syndrome coronavirus 2 was approved for use in adolescents in June 2021, shortly before an outbreak of B.1.617.2 (Delta) variant-dominant infection. We evaluated short-term vaccine effectiveness and found the vaccine to be highly effective among this population in this setting.
Keywords: 2019 novel coronavirus disease; COVID-19; Delta variant; Israel; SARS-CoV-2; adolescents; coronavirus disease; respiratory infections; severe acute respiratory syndrome coronavirus 2; vaccine effectiveness; viruses; zoonoses.
Figures
References
-
- Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes Pfizer-BioNTech COVID-19 vaccine for emergency use in adolescents in another important action in fight against pandemic [cited 2021 Aug 23]. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19...
-
- European Medicines Agency. First COVID-19 vaccine approved for children aged 12 to 15 in EU [cited 2021 Aug 23]. https://www.ema.europa.eu/en/news/first-covid-19-vaccine-approved-childr...
-
- Israel Ministry of Health. Ministry of Health's position regarding the expansion of the vaccination operation to ages 12–16 years [cited 2021 Aug 23]. https://www.gov.il/en/departments/news/02062021-01
-
- Israel Ministry of Health. Coronavirus outbreak in Binyamina [cited 2021 Aug 23]. https://www.gov.il/en/departments/news/19062021-02
-
- Israel Ministry of Health. Effective today (20.6.2021)—masking requirement at all schools in Modi’in and Binyamina [cited 2021 Aug 23]. https://www.gov.il/en/departments/news/20062021-01
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

