Co-culture of type I and type II pneumocytes as a model of alveolar epithelium
- PMID: 34570783
- PMCID: PMC8475999
- DOI: 10.1371/journal.pone.0248798
Co-culture of type I and type II pneumocytes as a model of alveolar epithelium
Abstract
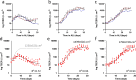
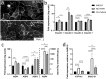
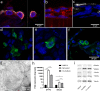
The epithelial tissues of the distal lung are continuously exposed to inhaled air, and are of research interest in studying respiratory exposure to both hazardous and therapeutic materials. Pharmaco-toxicological research depends on the development of sophisticated models of the alveolar epithelium, which better represent the different cell types present in the native lung and interactions between them. We developed an air-liquid interface (ALI) model of the alveolar epithelium which incorporates cell lines which bear features of type I (hAELVi) and type II (NCI-H441) epithelial cells. We compared morphology of single cells and the structure of cell layers of the two lines using light and electron microscopy. Working both in monotypic cultures and cocultures, we measured barrier function by trans-epithelial electrical resistance (TEER), and demonstrated that barrier properties can be maintained for 30 days. We created a mathematical model of TEER development over time based on these data in order to make inferences about the interactions occurring in these culture systems. We assessed expression of a panel of relevant genes that play important roles in barrier function and differentiation. The coculture model was observed to form a stable barrier akin to that seen in hAELVi, while expressing surfactant protein C, and having a profile of expression of claudins and aquaporins appropriate for the distal lung. We described cavities which arise within stratified cell layers in NCI-H441 and cocultured cells, and present evidence that these cavities represent an aberrant apical surface. In summary, our results support the coculture of these two cell lines to produce a model which better represents the breadth of functions seen in native alveolar epithelium.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Hynes J, Marshall L, Adcock I, Novotny T, Nic M, Dibusz K, et al.. Advanced Non-animal Models in Biomedical Research [Internet]. 2020. Available from: https://ec.europa.eu/jrc/en/publication/advanced-non-animal-models-biome...
-
- Costa A, de Souza Carvalho-Wodarz C, Seabra V, Sarmento B, Lehr CM. Triple co-culture of human alveolar epithelium, endothelium and macrophages for studying the interaction of nanocarriers with the air-blood barrier. Acta Biomater [Internet]. 2019;91:235–47. Available from: 10.1016/j.actbio.2019.04.037 - DOI - PubMed
-
- Kletting S. Co-culture of human alveolar epithelial (hAELVi) and macrophage (THP-1) cell lines. ALTEX [Internet]. 2018;211–22. Available from: https://www.altex.org/index.php/altex/article/view/89 doi: 10.14573/altex.1607191 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

