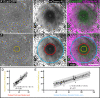
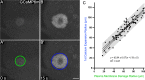
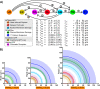
Zones of cellular damage around pulsed-laser wounds
- PMID: 34570791
- PMCID: PMC8476025
- DOI: 10.1371/journal.pone.0253032
Zones of cellular damage around pulsed-laser wounds
Abstract
After a tissue is wounded, cells surrounding the wound adopt distinct wound-healing behaviors to repair the tissue. Considerable effort has been spent on understanding the signaling pathways that regulate immune and tissue-resident cells as they respond to wounds, but these signals must ultimately originate from the physical damage inflicted by the wound. Tissue wounds comprise several types of cellular damage, and recent work indicates that different types of cellular damage initiate different types of signaling. Hence to understand wound signaling, it is important to identify and localize the types of wound-induced cellular damage. Laser ablation is widely used by researchers to create reproducible, aseptic wounds in a tissue that can be live-imaged. Because laser wounding involves a combination of photochemical, photothermal and photomechanical mechanisms, each with distinct spatial dependencies, cells around a pulsed-laser wound will experience a gradient of damage. Here we exploit this gradient to create a map of wound-induced cellular damage. Using genetically-encoded fluorescent proteins, we monitor damaged cellular and sub-cellular components of epithelial cells in living Drosophila pupae in the seconds to minutes following wounding. We hypothesized that the regions of damage would be predictably arrayed around wounds of varying sizes, and subsequent analysis found that all damage radii are linearly related over a 3-fold range of wound size. Thus, around laser wounds, the distinct regions of damage can be estimated after measuring any one. This report identifies several different types of cellular damage within a wounded epithelial tissue in a living animal. By quantitatively mapping the size and placement of these different types of damage, we set the foundation for tracing wound-induced signaling back to the damage that initiates it.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Multiple Mechanisms Drive Calcium Signal Dynamics around Laser-Induced Epithelial Wounds.Biophys J. 2017 Oct 3;113(7):1623-1635. doi: 10.1016/j.bpj.2017.07.022. Biophys J. 2017. PMID: 28978452 Free PMC article.
-
Skin graft take and healing following 193-nm excimer, continuous-wave carbon dioxide (CO2), pulsed CO2, or pulsed holmium: YAG laser ablation of the graft bed.Arch Dermatol. 1993 Aug;129(8):979-88. Arch Dermatol. 1993. PMID: 8352622
-
Comparison of carbon dioxide laser, erbium:YAG laser, dermabrasion, and dermatome: a study of thermal damage, wound contraction, and wound healing in a live pig model: implications for skin resurfacing.J Am Acad Dermatol. 2000 Jan;42(1 Pt 1):92-105. doi: 10.1016/s0190-9622(00)90016-1. J Am Acad Dermatol. 2000. PMID: 10607327
-
Pathologic analysis of photothermal and photomechanical effects of laser-tissue interactions.Photochem Photobiol. 1991 Jun;53(6):825-35. doi: 10.1111/j.1751-1097.1991.tb09897.x. Photochem Photobiol. 1991. PMID: 1886941 Review.
-
Wound Healing and Cell Dynamics Including Mesenchymal and Dental Pulp Stem Cells Induced by Photobiomodulation Therapy: An Example of Socket-Preserving Effects after Tooth Extraction in Rats and a Literature Review.Int J Mol Sci. 2020 Sep 18;21(18):6850. doi: 10.3390/ijms21186850. Int J Mol Sci. 2020. PMID: 32961958 Free PMC article. Review.
Cited by
-
Understanding the diversity and dynamics of in vivo efferocytosis: Insights from the fly embryo.Immunol Rev. 2023 Oct;319(1):27-44. doi: 10.1111/imr.13266. Epub 2023 Aug 17. Immunol Rev. 2023. PMID: 37589239 Free PMC article. Review.
-
After wounding, a G-protein coupled receptor promotes the restoration of tension in epithelial cells.bioRxiv [Preprint]. 2024 Feb 18:2023.05.31.543122. doi: 10.1101/2023.05.31.543122. bioRxiv. 2024. Update in: Mol Biol Cell. 2024 May 1;35(5):ar66. doi: 10.1091/mbc.E23-05-0204. PMID: 37398151 Free PMC article. Updated. Preprint.
-
Mounting Drosophila pupae for laser ablation and live imaging of the dorsal thorax.STAR Protoc. 2022 May 14;3(2):101396. doi: 10.1016/j.xpro.2022.101396. eCollection 2022 Jun 17. STAR Protoc. 2022. PMID: 35600923 Free PMC article.
-
Ferroptosis-like cell death promotes and prolongs inflammation in Drosophila.Nat Cell Biol. 2024 Sep;26(9):1535-1544. doi: 10.1038/s41556-024-01450-7. Epub 2024 Jun 25. Nat Cell Biol. 2024. PMID: 38918597 Free PMC article.
-
Engineering tools for quantifying and manipulating forces in epithelia.Biophys Rev (Melville). 2023 May 11;4(2):021303. doi: 10.1063/5.0142537. eCollection 2023 Jun. Biophys Rev (Melville). 2023. PMID: 38510344 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

