Autologous transplant vs chimeric antigen receptor T-cell therapy for relapsed DLBCL in partial remission
- PMID: 34570879
- PMCID: PMC8900276
- DOI: 10.1182/blood.2021013289
Autologous transplant vs chimeric antigen receptor T-cell therapy for relapsed DLBCL in partial remission
Abstract
The relative efficacy of autologous hematopoietic cell transplant (auto-HCT) vs chimeric antigen receptor T-cell (CAR-T) therapy in patients with diffuse large B-cell lymphoma (DLBCL) who achieve a partial remission (PR) after salvage chemotherapy is not known. Using the Center for International Blood & Marrow Transplant Research registry database, we identified adult patients with DLBCL who received either an auto-HCT (2013-2019) or CAR-T treatment with axicabtagene ciloleucel (2018-2019) while in a PR by computed tomography or positron emission tomography scan. We compared the clinical outcomes between the 2 cohorts using univariable and multivariable regression models after adjustment for relevant baseline and clinical factors. In the univariable analysis, the 2-year progression-free survival (52% vs 42%; P = .1) and the rate of 100-day nonrelapse mortality (4% vs 2%; P = .3) were not different between the 2 cohorts, but consolidation with auto-HCT was associated with a lower rate of relapse/progression (40% vs 53%; P = .05) and a superior overall survival (OS) (69% vs 47%; P = .004) at 2 years. In the multivariable regression analysis, treatment with auto-HCT was associated with a significantly lower risk of relapse/progression rate (hazard ratio = 1.49; P = .01) and a superior OS (hazard ratio = 1.63; P = .008). In patients with DLBCL in a PR after salvage therapy, treatment with auto-HCT was associated with a lower incidence of relapse and a superior OS compared with CAR-T. These data support the role of auto-HCT as the standard of care in transplant-eligible patients with relapsed DLBCL in PR after salvage therapy.
© 2022 by The American Society of Hematology.
Figures

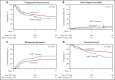
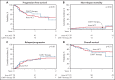
Comment in
-
CAR T cells and autologous transplantation can coexist for DLBCL.Blood. 2022 Mar 3;139(9):1266-1267. doi: 10.1182/blood.2021014066. Blood. 2022. PMID: 35238887 Free PMC article. No abstract available.
-
ASCT vs CART for patients with relapsed LBCL in PR: role of TMTV.Blood Adv. 2023 Jun 13;7(11):2586-2589. doi: 10.1182/bloodadvances.2022009622. Blood Adv. 2023. PMID: 36745104 Free PMC article. No abstract available.
References
-
- Kanate AS, Majhail NS, Savani BN, et al. . Indications for hematopoietic cell transplantation and immune effector cell therapy: guidelines from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transplant. 2020;26(7):1247-1256. - PubMed
-
- Philip T, Guglielmi C, Hagenbeek A, et al. . Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333(23):1540-1545. - PubMed
-
- Abramson JS, Palomba ML, Gordon LI, et al. . Lisocabtagene Maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020; 396(10254):839-852. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

