Dynamics of Reverse Transcription-Polymerase Chain Reaction and Serologic Test Results in Children with SARS-CoV-2 Infection
- PMID: 34571020
- PMCID: PMC8463102
- DOI: 10.1016/j.jpeds.2021.09.029
Dynamics of Reverse Transcription-Polymerase Chain Reaction and Serologic Test Results in Children with SARS-CoV-2 Infection
Abstract
Objectives: To determine the time to reverse transcription-polymerase chain reaction (RT-PCR) negativity after the first positive RT-PCR test, factors associated with longer time to RT-PCR negativity, proportion of children seroconverting after proven severe acute respiratory syndrome coronavirus 2 infection, and factors associated with the lack of seroconversion.
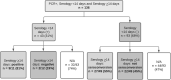
Study design: The Epidemiological Study of Coronavirus in Children of the Spanish Society of Pediatrics is a multicenter study conducted in Spanish children to assess the characteristics of coronavirus disease 2019. In a subset of patients, 3 serial RT-PCR tests on nasopharyngeal swab specimens were performed after the first RT-PCR test, and immunoglobulin G serology for severe acute respiratory syndrome coronavirus 2 antibodies was performed in the acute and follow-up (<14 and ≥14 days after diagnosis) phase.
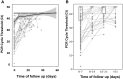
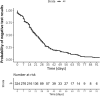
Results: In total, 324 patients were included in the study. The median time to RT-PCR negativity was 17 days (IQR, 8-29 days), and 35% of patients remained positive more than 4 weeks after the first RT-PCR test. The probability of RT-PCR negativity did not differ across groups defined by sex, disease severity, immunosuppressive drugs, or clinical phenotype. Globally, 24% of children failed to seroconvert after infection. Seroconversion was associated with hospitalization, persistence of RT-PCR positivity, and days of fever.
Conclusions: Time to RT-PCR negativity was long, regardless of the severity of symptoms or other patient features. This finding should be considered when interpreting RT-PCR results in a child with symptoms, especially those with mild symptoms. Seroprevalence and postimmunization studies should consider that 11 in 4 infected children fail to seroconvert.
Keywords: COVID-19; RT-PCR; SARS-CoV-2; children; dynamics; seroconversion; serology.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures

References
-
- Long Q.-X., Tang X.-J., Shi Q.-L., Li Q., Deng H.-J., Yuan J., et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–1204. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

