The combined treatment of Molnupiravir and Favipiravir results in a potentiation of antiviral efficacy in a SARS-CoV-2 hamster infection model
- PMID: 34571361
- PMCID: PMC8461366
- DOI: 10.1016/j.ebiom.2021.103595
The combined treatment of Molnupiravir and Favipiravir results in a potentiation of antiviral efficacy in a SARS-CoV-2 hamster infection model
Abstract
Background: Favipiravir and Molnupiravir, orally available antivirals, have been reported to exert antiviral activity against SARS-CoV-2. First efficacy data have been recently reported in COVID-19 patients.
Methods: We here report on the combined antiviral effect of both drugs in a SARS-CoV-2 Syrian hamster infection model. The infected hamsters were treated twice daily with the vehicle (the control group) or a suboptimal dose of each compound or a combination of both compounds.
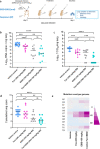
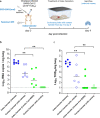
Findings: When animals were treated with a combination of suboptimal doses of Molnupiravir and Favipiravir at the time of infection, a marked combined potency at endpoint is observed. Infectious virus titers in the lungs of animals treated with the combination are reduced by ∼5 log10 and infectious virus are no longer detected in the lungs of >60% of treated animals. When start of treatment was delayed with one day a reduction of titers in the lungs of 2.4 log10 was achieved. Moreover, treatment of infected animals nearly completely prevented transmission to co-housed untreated sentinels. Both drugs result in an increased mutation frequency of the remaining viral RNA recovered from the lungs of treated animals. In the combo-treated hamsters, an increased frequency of C-to-T mutations in the viral RNA is observed as compared to the single treatment groups which may explain the pronounced antiviral potency of the combination.
Interpretation: Our findings may lay the basis for the design of clinical studies to test the efficacy of the combination of Molnupiravir/Favipiravir in the treatment of COVID-19.
Funding: stated in the acknowledgment.
Keywords: Antivirals; Favipiravir, hamsters, coronavirus; Molnupiravir; SARS-CoV-2.
Copyright © 2021 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest None to declare.
Figures

References
-
- Kaptein SJF, Jacobs S, Langendries L, Seldeslachts L, ter Horst S, Liesenborghs L. Favipiravir at high doses has potent antiviral activity in SARS-CoV-2−infected hamsters, whereas hydroxychloroquine lacks activity. Proc Natl Acad Sci U S A. 2020;117:26955–26965. doi: 10.1073/pnas.2014441117. - DOI - PMC - PubMed
-
- Yakubova E, Ivashchenko A, Sitdekov T, Egorova A, Merkulova E, Blinow A. Multicenter, open-labeled efficacy study of Avifavir in patients with COVID-19. Conf. Retroviruses Opportunistic Infect. Virtual. 2021
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

