Temperatures Outside the Optimal Range for Helicobacter pylori Increase Its Harboring within Candida Yeast Cells
- PMID: 34571792
- PMCID: PMC8472035
- DOI: 10.3390/biology10090915
Temperatures Outside the Optimal Range for Helicobacter pylori Increase Its Harboring within Candida Yeast Cells
Abstract
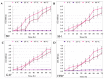
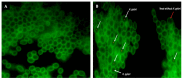
Helicobacter pylori is capable of entering into yeast, but the factors driving this endosymbiosis remain unknown. This work aimed to determine if temperatures outside the optimal range for H. pylori increase its harboring within Candida. H. pylori strains were co-cultured with Candida strains in Brucella broth supplemented with 5% fetal bovine serum and incubated at 4, 25, 37 or 40 °C. After co-culturing, yeasts containing bacteria-like bodies (Y-BLBs) were observed by optical microscopy, and the bacterium were identified as H. pylori by FISH. The H. pylori 16S rRNA gene was amplified from the total DNA of Y-BLBs. The viability of intra-yeast H. pylori cells was confirmed using a viability assay. All H. pylori strains were capable of entering into all Candida strains assayed. The higher percentages of Y-BLBs are obtained at 40 °C with any of the Candida strains. H pylori also increased its harboring within yeast in co-cultures incubated at 25 °C when compared to those incubated at 37 °C. In conclusion, although H. pylori grew significantly at 40 °C, this temperature increased its harboring within Candida. The endosymbiosis between both microorganisms is strain-dependent and permits bacterial cells to remain viable under the stressing environmental conditions assayed.
Keywords: Candida; Helicobacter pylori; intracellular; stress; temperature.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
An Anaerobic Environment Drives the Harboring of Helicobacter pylori within Candida Yeast Cells.Biology (Basel). 2022 May 12;11(5):738. doi: 10.3390/biology11050738. Biology (Basel). 2022. PMID: 35625466 Free PMC article.
-
Nutrient Deficiency Promotes the Entry of Helicobacter pylori Cells into Candida Yeast Cells.Biology (Basel). 2021 May 12;10(5):426. doi: 10.3390/biology10050426. Biology (Basel). 2021. PMID: 34065788 Free PMC article.
-
Candida albicans Release Intracellular Bacteria When Treated With Amphotericin B.Arch Iran Med. 2018 May 1;21(5):191-198. Arch Iran Med. 2018. PMID: 29738262
-
In Vitro Incorporation of Helicobacter pylori into Candida albicans Caused by Acidic pH Stress.Pathogens. 2020 Jun 19;9(6):489. doi: 10.3390/pathogens9060489. Pathogens. 2020. PMID: 32575493 Free PMC article.
-
Immunodetection of Helicobacter pylori-specific proteins in oral and gastric Candida yeasts.Arch Iran Med. 2013 Nov;16(11):624-30. Arch Iran Med. 2013. PMID: 24206402
Cited by
-
An Anaerobic Environment Drives the Harboring of Helicobacter pylori within Candida Yeast Cells.Biology (Basel). 2022 May 12;11(5):738. doi: 10.3390/biology11050738. Biology (Basel). 2022. PMID: 35625466 Free PMC article.
-
Polymicrobial interactions of Helicobacter pylori and its role in the process of oral diseases.J Oral Microbiol. 2025 Feb 25;17(1):2469896. doi: 10.1080/20002297.2025.2469896. eCollection 2025. J Oral Microbiol. 2025. PMID: 40013013 Free PMC article. Review.
-
In Search for Reasons behind Helicobacter pylori Eradication Failure-Assessment of the Antibiotics Resistance Rate and Co-Existence of Helicobacter pylori with Candida Species.J Fungi (Basel). 2023 Mar 7;9(3):328. doi: 10.3390/jof9030328. J Fungi (Basel). 2023. PMID: 36983496 Free PMC article.
-
Gastric microbiota dysbiosis and Helicobacter pylori infection.Front Microbiol. 2023 Mar 30;14:1153269. doi: 10.3389/fmicb.2023.1153269. eCollection 2023. Front Microbiol. 2023. PMID: 37065152 Free PMC article. Review.
-
Surface adherence and vacuolar internalization of bacterial pathogens to the Candida spp. cells: Mechanism of persistence and propagation.J Adv Res. 2023 Nov;53:115-136. doi: 10.1016/j.jare.2022.12.013. Epub 2022 Dec 23. J Adv Res. 2023. PMID: 36572338 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

