Annexin A1 as a Regulator of Immune Response in Cancer
- PMID: 34571894
- PMCID: PMC8464935
- DOI: 10.3390/cells10092245
Annexin A1 as a Regulator of Immune Response in Cancer
Abstract
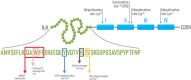
Annexin A1 is a 37 kDa phospholipid-binding protein that is expressed in many tissues and cell types, including leukocytes, lymphocytes and epithelial cells. Although Annexin A1 has been extensively studied for its anti-inflammatory activity, it has been shown that, in the cancer context, its activity switches from anti-inflammatory to pro-inflammatory. Remarkably, Annexin A1 shows pro-invasive and pro-tumoral properties in several cancers either by eliciting autocrine signaling in cancer cells or by inducing a favorable tumor microenvironment. Indeed, the signaling of the N-terminal peptide of AnxA1 has been described to promote the switching of macrophages to the pro-tumoral M2 phenotype. Moreover, AnxA1 has been described to prevent the induction of antigen-specific cytotoxic T cell response and to play an essential role in the induction of regulatory T lymphocytes. In this way, Annexin A1 inhibits the anti-tumor immunity and supports the formation of an immunosuppressed tumor microenvironment that promotes tumor growth and metastasis. For these reasons, in this review we aim to describe the role of Annexin A1 in the establishment of the tumor microenvironment, focusing on the immunosuppressive and immunomodulatory activities of Annexin A1 and on its interaction with the epidermal growth factor receptor.
Keywords: Annexin A1; cancer aggressiveness; immune-suppression; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

