HCV-Induced Immunometabolic Crosstalk in a Triple-Cell Co-Culture Model Capable of Simulating Systemic Iron Homeostasis
- PMID: 34571900
- PMCID: PMC8465420
- DOI: 10.3390/cells10092251
HCV-Induced Immunometabolic Crosstalk in a Triple-Cell Co-Culture Model Capable of Simulating Systemic Iron Homeostasis
Abstract
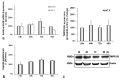
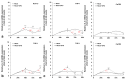
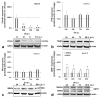
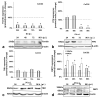
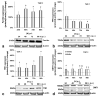
Iron is crucial to the regulation of the host innate immune system and the outcome of many infections. Hepatitis C virus (HCV), one of the major viral human pathogens that depends on iron to complete its life cycle, is highly skilled in evading the immune system. This study presents the construction and validation of a physiologically relevant triple-cell co-culture model that was used to investigate the input of iron in HCV infection and the interplay between HCV, iron, and determinants of host innate immunity. We recorded the expression patterns of key proteins of iron homeostasis involved in iron import, export and storage and examined their relation to the iron regulatory hormone hepcidin in hepatocytes, enterocytes and macrophages in the presence and absence of HCV. We then assessed the transcriptional profiles of pro-inflammatory cytokines Interleukin-6 (IL-6) and interleukin-15 (IL-15) and anti-inflammatory interleukin-10 (IL-10) under normal or iron-depleted conditions and determined how these were affected by infection. Our data suggest the presence of a link between iron homeostasis and innate immunity unfolding among liver, intestine, and macrophages, which could participate in the deregulation of innate immune responses observed in early HCV infection. Coupled with iron-assisted enhanced viral propagation, such a mechanism may be important for the establishment of viral persistence and the ensuing chronic liver disease.
Keywords: HCV; co-culture; cytokines; enterocytes; ferritin; hepcidin; innate immunity; iron; macrophages.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
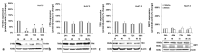
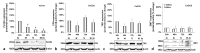
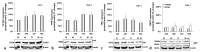


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

