Presynaptic AMPA Receptors in Health and Disease
- PMID: 34571906
- PMCID: PMC8470629
- DOI: 10.3390/cells10092260
Presynaptic AMPA Receptors in Health and Disease
Abstract
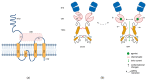
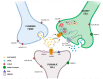
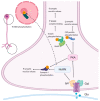
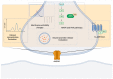
AMPA receptors (AMPARs) are ionotropic glutamate receptors that play a major role in excitatory neurotransmission. AMPARs are located at both presynaptic and postsynaptic plasma membranes. A huge number of studies investigated the role of postsynaptic AMPARs in the normal and abnormal functioning of the mammalian central nervous system (CNS). These studies highlighted that changes in the functional properties or abundance of postsynaptic AMPARs are major mechanisms underlying synaptic plasticity phenomena, providing molecular explanations for the processes of learning and memory. Conversely, the role of AMPARs at presynaptic terminals is as yet poorly clarified. Accruing evidence demonstrates that presynaptic AMPARs can modulate the release of various neurotransmitters. Recent studies also suggest that presynaptic AMPARs may possess double ionotropic-metabotropic features and that they are involved in the local regulation of actin dynamics in both dendritic and axonal compartments. In addition, evidence suggests a key role of presynaptic AMPARs in axonal pathology, in regulation of pain transmission and in the physiology of the auditory system. Thus, it appears that presynaptic AMPARs play an important modulatory role in nerve terminal activity, making them attractive as novel pharmacological targets for a variety of pathological conditions.
Keywords: GABA; acetylcholine; catecholamines; glutamate; serotonin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

