Free Transplantation of a Tissue Engineered Bone Graft into an Irradiated, Critical-Size Femoral Defect in Rats
- PMID: 34571907
- PMCID: PMC8467400
- DOI: 10.3390/cells10092256
Free Transplantation of a Tissue Engineered Bone Graft into an Irradiated, Critical-Size Femoral Defect in Rats
Abstract
Healing of large bone defects remains a challenge in reconstructive surgery, especially with impaired healing potential due to severe trauma, infection or irradiation. In vivo studies are often performed in healthy animals, which might not accurately reflect the situation in clinical cases. In the present study, we successfully combined a critical-sized femoral defect model with an ionizing radiation protocol in rats. To support bone healing, tissue-engineered constructs were transferred into the defect after ectopic preossification and prevascularization. The combination of SiHA, MSCs and BMP-2 resulted in the significant ectopic formation of bone tissue, which can easily be transferred by means of our custom-made titanium chamber. Implanted osteogenic MSCs survived in vivo for a total of 18 weeks. The use of SiHA alone did not lead to bone formation after ectopic implantation. Analysis of gene expression showed early osteoblast differentiation and a hypoxic and inflammatory environment in implanted constructs. Irradiation led to impaired bone healing, decreased vascularization and lower short-term survival of implanted cells. We conclude that our model is highly valuable for the investigation of bone healing and tissue engineering in pre-damaged tissue and that healing of bone defects can be substantially supported by combining SiHA, MSCs and BMP-2.
Keywords: bone tissue engineering; critical size defect; hydroxyapatite; irradiation; mesenchymal stem cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

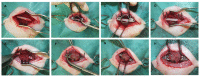

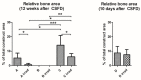




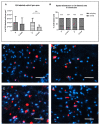
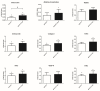
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

