Biosynthesis of the Novel Endogenous 15-Lipoxygenase Metabolites N-13-Hydroxy-octodecadienoyl-ethanolamine and 13-Hydroxy-octodecadienoyl-glycerol by Human Neutrophils and Eosinophils
- PMID: 34571971
- PMCID: PMC8470279
- DOI: 10.3390/cells10092322
Biosynthesis of the Novel Endogenous 15-Lipoxygenase Metabolites N-13-Hydroxy-octodecadienoyl-ethanolamine and 13-Hydroxy-octodecadienoyl-glycerol by Human Neutrophils and Eosinophils
Abstract
The endocannabinoids 2-arachidonoyl-glycerol and N-arachidonoyl-ethanolamine are lipids regulating many physiological processes, notably inflammation. Endocannabinoid hydrolysis inhibitors are now being investigated as potential anti-inflammatory agents. In addition to 2-arachidonoyl-glycerol and N-arachidonoyl-ethanolamine, the endocannabinoidome also includes other monoacylglycerols and N-acyl-ethanolamines such as 1-linoleoyl-glycerol (1-LG) and N-linoleoyl-ethanolamine (LEA). By increasing monoacylglycerols and/or N-acyl-ethanolamine levels, endocannabinoid hydrolysis inhibitors will likely increase the levels of their metabolites. Herein, we investigated whether 1-LG and LEA were substrates for the 15-lipoxygenase pathway, given that both possess a 1Z,4Z-pentadiene motif, near their omega end. We thus assessed how human eosinophils and neutrophils biosynthesized the 15-lipoxygenase metabolites of 1-LG and LEA. Linoleic acid (LA), a well-documented substrate of 15-lipoxygenases, was used as positive control. N-13-hydroxy-octodecadienoyl-ethanolamine (13-HODE-EA) and 13-hydroxy-octodecadienoyl-glycerol (13-HODE-G), the 15-lipoxygenase metabolites of LEA and 1-LG, were synthesized using Novozym 435 and soybean lipoxygenase. Eosinophils, which express the 15-lipoxygenase-1, metabolized LA, 1-LG, and LEA into their 13-hydroxy derivatives. This was almost complete after five minutes. Substrate preference of eosinophils was LA > LEA > 1-LG in presence of 13-HODE-G hydrolysis inhibition with methyl-arachidonoyl-fluorophosphonate. Human neutrophils also metabolized LA, 1-LG, and LEA into their 13-hydroxy derivatives. This was maximal after 15-30 s. Substrate preference was LA ≫ 1-LG > LEA. Importantly, 13-HODE-G was found in humans and mouse tissue samples. In conclusion, our data show that human eosinophils and neutrophils metabolize 1-LG and LEA into the novel endogenous 15-lipoxygenase metabolites 13-HODE-G and 13-HODE-EA. The full biological importance of 13-HODE-G and 13-HODE-EA remains to be explored.
Keywords: 13-HODE; 2-arachidonoyl-glycerol; N-linoleoyl-ethanolamine; anandamide; eicosanoid; endocannabinoid; eosinophils; linoleic acid; linoleoyl-glycerol; neutrophils.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



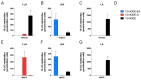




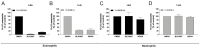
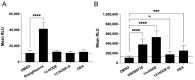

References
-
- Xie S., Borazjani A., Hatfield M.J., Edwards C.C., Potter P.M., Ross M.K. Inactivation of lipid glyceryl ester metabolism in human THP1 monocytes/macrophages by activated organophosphorus insecticides: Role of carboxylesterases 1 and 2. Chem. Res. Toxicol. 2010;23:1890–1904. doi: 10.1021/tx1002194. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

