Regulation of T Cell Responses by Ionic Salt Signals
- PMID: 34572015
- PMCID: PMC8471541
- DOI: 10.3390/cells10092365
Regulation of T Cell Responses by Ionic Salt Signals
Abstract
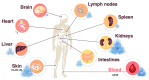
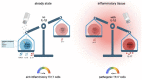
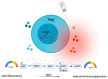
T helper cell responses are tailored to their respective antigens and adapted to their specific tissue microenvironment. While a great proportion of T cells acquire a resident identity, a significant proportion of T cells continue circulating, thus encountering changing microenvironmental signals during immune surveillance. One signal, which has previously been largely overlooked, is sodium chloride. It has been proposed to have potent effects on T cell responses in the context of autoimmune, allergic and infectious tissue inflammation in mouse models and humans. Sodium chloride is stringently regulated in the blood by the kidneys but displays differential deposition patterns in peripheral tissues. Sodium chloride accumulation might furthermore be regulated by dietary intake and thus by intentional behavior. Together, these results make sodium chloride an interesting but still controversial signal for immune modulation. Its downstream cellular activities represent a potential therapeutic target given its effects on T cell cytokine production. In this review article, we provide an overview and critical evaluation of the impact of this ionic signal on T helper cell polarization and T helper cell effector functions. In addition, the impact of sodium chloride from the tissue microenvironment is assessed for human health and disease and for its therapeutic potential.
Keywords: T cells; allergy; autoimmunity; cytokines; immune regulation; sodium chloride.
Conflict of interest statement
The author declares no conflict of interest.
Figures
Similar articles
-
Sodium chloride is an ionic checkpoint for human TH2 cells and shapes the atopic skin microenvironment.Sci Transl Med. 2019 Feb 20;11(480):eaau0683. doi: 10.1126/scitranslmed.aau0683. Sci Transl Med. 2019. PMID: 30787167
-
Critical Roles of Balanced T Helper 9 Cells and Regulatory T Cells in Allergic Airway Inflammation and Tumor Immunity.J Immunol Res. 2021 Mar 1;2021:8816055. doi: 10.1155/2021/8816055. eCollection 2021. J Immunol Res. 2021. PMID: 33748292 Free PMC article. Review.
-
Impact of combined sodium chloride and saturated long-chain fatty acid challenge on the differentiation of T helper cells in neuroinflammation.J Neuroinflammation. 2017 Sep 12;14(1):184. doi: 10.1186/s12974-017-0954-y. J Neuroinflammation. 2017. PMID: 28899400 Free PMC article.
-
T helper cells plasticity in inflammation.Cytometry A. 2014 Jan;85(1):36-42. doi: 10.1002/cyto.a.22348. Epub 2013 Sep 5. Cytometry A. 2014. PMID: 24009159 Review.
-
Interplay between effector Th17 and regulatory T cells.J Clin Immunol. 2008 Nov;28(6):660-70. doi: 10.1007/s10875-008-9239-7. Epub 2008 Sep 23. J Clin Immunol. 2008. PMID: 18810613
Cited by
-
A High-Sodium Diet Modulates the Immune Response of Food Allergy in a Murine Model.Nutrients. 2021 Oct 20;13(11):3684. doi: 10.3390/nu13113684. Nutrients. 2021. PMID: 34835940 Free PMC article.
-
Oxidative Stress Induced by High Salt Diet-Possible Implications for Development and Clinical Manifestation of Cutaneous Inflammation and Endothelial Dysfunction in Psoriasis vulgaris.Antioxidants (Basel). 2022 Jun 27;11(7):1269. doi: 10.3390/antiox11071269. Antioxidants (Basel). 2022. PMID: 35883760 Free PMC article. Review.
-
Electrolyte imbalance causes suppression of NK and T cell effector function in malignant ascites.J Exp Clin Cancer Res. 2023 Sep 8;42(1):235. doi: 10.1186/s13046-023-02798-8. J Exp Clin Cancer Res. 2023. PMID: 37684704 Free PMC article.
References
-
- Veldhoen M., Uyttenhove C., Van Snick J., Helmby H., Westendorf A., Buer J., Martin B., Wilhelm C., Stockinger B. Transforming growth factor-β ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9–producing subset. Nat. Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. - DOI - PubMed
-
- Mosmann T.R., Cherwinski H., Bond M.W., Giedlin M.A., Coffman R.L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986;136:2348–2357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

