Sex-Dependent Effects of Intestinal Microbiome Manipulation in a Mouse Model of Alzheimer's Disease
- PMID: 34572019
- PMCID: PMC8469717
- DOI: 10.3390/cells10092370
Sex-Dependent Effects of Intestinal Microbiome Manipulation in a Mouse Model of Alzheimer's Disease
Abstract
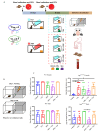
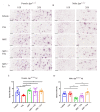
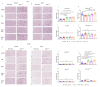
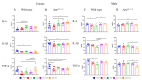
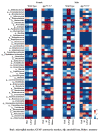
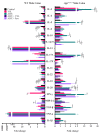
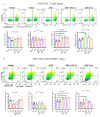
Mechanisms linking intestinal bacteria and neurodegenerative diseases such as Alzheimer's disease (AD) are still unclear. We hypothesized that intestinal dysbiosis might potentiate AD, and manipulating the microbiome to promote intestinal eubiosis and immune homeostasis may improve AD-related brain changes. This study assessed sex differences in the effects of oral probiotic, antibiotics, and synbiotic treatments in the AppNL-G-F mouse model of AD. The fecal microbiome demonstrated significant correlations between bacterial genera in AppNL-G-F mice and Aβ plaque load, gliosis, and memory performance. Female and not male AppNL-G-F mice fed probiotic but not synbiotic exhibited a decrease in Aβ plaques, microgliosis, brain TNF-α, and memory improvement compared to no treatment controls. Although antibiotics treatment did not produce these multiple changes in brain cytokines, memory, or gliosis, it did decrease Aβ plaque load and colon cytokines in AppNL-G-F males. The intestinal cytokine milieu and splenocyte phenotype of female but not male AppNL-G-F mice indicated a modest proinflammatory innate response following probiotic treatment compared to controls, with an adaptive response following antibiotics treatment in male AppNL-G-F mice. Overall, these results demonstrate the beneficial effects of probiotic only in AppNL-G-F females, with minimal benefits of antibiotics or synbiotic feeding in male or female mice.
Keywords: Alzheimer’s disease; VSL#3; antibiotics; cytokines; immune response; microbiome; prebiotic; probiotic; splenocytes; synbiotic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

