HIF-1-Independent Mechanisms Regulating Metabolic Adaptation in Hypoxic Cancer Cells
- PMID: 34572020
- PMCID: PMC8472468
- DOI: 10.3390/cells10092371
HIF-1-Independent Mechanisms Regulating Metabolic Adaptation in Hypoxic Cancer Cells
Abstract
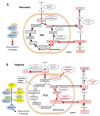
In solid tumours, cancer cells exist within hypoxic microenvironments, and their metabolic adaptation to this hypoxia is driven by HIF-1 transcription factor, which is overexpressed in a broad range of human cancers. HIF inhibitors are under pre-clinical investigation and clinical trials, but there is evidence that hypoxic cancer cells can adapt metabolically to HIF-1 inhibition, which would provide a potential route for drug resistance. Here, we review accumulating evidence of such adaptions in carbohydrate and creatine metabolism and other HIF-1-independent mechanisms that might allow cancers to survive hypoxia despite anti-HIF-1 therapy. These include pathways in glucose, glutamine, and lipid metabolism; epigenetic mechanisms; post-translational protein modifications; spatial reorganization of enzymes; signalling pathways such as Myc, PI3K-Akt, 2-hyxdroxyglutarate and AMP-activated protein kinase (AMPK); and activation of the HIF-2 pathway. All of these should be investigated in future work on hypoxia bypass mechanisms in anti-HIF-1 cancer therapy. In principle, agents targeted toward HIF-1β rather than HIF-1α might be advantageous, as both HIF-1 and HIF-2 require HIF-1β for activation. However, HIF-1β is also the aryl hydrocarbon nuclear transporter (ARNT), which has functions in many tissues, so off-target effects should be expected. In general, cancer therapy by HIF inhibition will need careful attention to potential resistance mechanisms.
Keywords: 2-hydroxyglutarate; AMP-activated protein kinase (AMPK); Myc; cancer metabolism; creatine metabolism; glutamine metabolism; glycolysis; hypoxia; hypoxia-inducible factor-1 (HIF-1); lipid metabolism; phosphatidylinositol 3-kinase (PI3K).
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

