Effects of Extracellular Osteoanabolic Agents on the Endogenous Response of Osteoblastic Cells
- PMID: 34572032
- PMCID: PMC8471159
- DOI: 10.3390/cells10092383
Effects of Extracellular Osteoanabolic Agents on the Endogenous Response of Osteoblastic Cells
Abstract
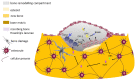
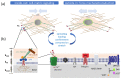
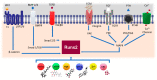
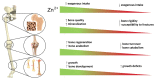
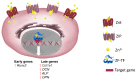
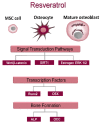
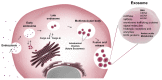
The complex multidimensional skeletal organization can adapt its structure in accordance with external contexts, demonstrating excellent self-renewal capacity. Thus, optimal extracellular environmental properties are critical for bone regeneration and inextricably linked to the mechanical and biological states of bone. It is interesting to note that the microstructure of bone depends not only on genetic determinants (which control the bone remodeling loop through autocrine and paracrine signals) but also, more importantly, on the continuous response of cells to external mechanical cues. In particular, bone cells sense mechanical signals such as shear, tensile, loading and vibration, and once activated, they react by regulating bone anabolism. Although several specific surrounding conditions needed for osteoblast cells to specifically augment bone formation have been empirically discovered, most of the underlying biomechanical cellular processes underneath remain largely unknown. Nevertheless, exogenous stimuli of endogenous osteogenesis can be applied to promote the mineral apposition rate, bone formation, bone mass and bone strength, as well as expediting fracture repair and bone regeneration. The following review summarizes the latest studies related to the proliferation and differentiation of osteoblastic cells, enhanced by mechanical forces or supplemental signaling factors (such as trace metals, nutraceuticals, vitamins and exosomes), providing a thorough overview of the exogenous osteogenic agents which can be exploited to modulate and influence the mechanically induced anabolism of bone. Furthermore, this review aims to discuss the emerging role of extracellular stimuli in skeletal metabolism as well as their potential roles and provide new perspectives for the treatment of bone disorders.
Keywords: antioxidant supplements; bone remodeling; exosomes; mechanically induced anabolism; ossification stimuli; osteoanabolic agents; osteoporosis; resveratrol; retinoic acid; zinc.
Conflict of interest statement
There are no potential conflict of interest relating to the manuscript for each author, and there were no extramural sources supporting this research (excluding sources already declared).
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

