Inflammasomes in the Pathophysiology of Aortic Disease
- PMID: 34572082
- PMCID: PMC8468335
- DOI: 10.3390/cells10092433
Inflammasomes in the Pathophysiology of Aortic Disease
Abstract
Aortic diseases comprise aneurysms, dissections, and several other pathologies. In general, aging is associated with a slow but progressive dilation of the aorta, along with increased stiffness and pulse pressure. The progression of aortic disease is characterized by subclinical development or acute presentation. Recent evidence suggests that inflammation participates causally in different clinical manifestations of aortic diseases. As of yet, diagnostic imaging and surveillance is mainly based on ultrasonography, computed tomography (CT), and magnetic resonance imaging (MRI). Little medical therapy is available so far to prevent or treat the majority of aortic diseases. Endovascular therapy by the introduction of covered stentgrafts provides the main treatment option, although open surgery and implantation of synthetic grafts remain necessary in many situations. Because of the risks associated with surgery, there is a need for identification of pharmaceutical targets interfering with the pathophysiology of aortic remodeling. The participation of innate immunity and inflammasome activation in different cell types is common in aortic diseases. This review will thus focus on inflammasome activities in vascular cells of different chronic and acute aortic diseases and discuss their role in development and progression. We will also identify research gaps and suggest promising therapeutic targets, which may be used for future medical interventions.
Keywords: AIM2; IMH; NLRP3; PAU; aneurysm; aorta; aortitis; dissection; inflammasome.
Conflict of interest statement
D.B. is a consultant, scientific advisor or involved in clinical trials for Medtronic, Gore, Cook Medical, Brainlab, Siemens. The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures
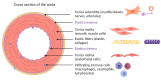


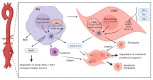
References
-
- Sampson U.K., Fowkes F.G., McDermott M.M., Criqui M.H., Aboyans V., Norman P.E., Forouzanfar M.H., Naghavi M., Song Y., Harrell F.E., Jr., et al. Global and regional burden of death and disability from peripheral artery disease: 21 world regions, 1990 to 2010. Glob. Heart. 2014;9:145–158.e121. doi: 10.1016/j.gheart.2013.12.008. - DOI - PubMed
-
- Sampson U.K., Norman P.E., Fowkes F.G., Aboyans V., Song Y., Harrell F.E., Jr., Forouzanfar M.H., Naghavi M., Denenberg J.O., McDermott M.M., et al. Estimation of global and regional incidence and prevalence of abdominal aortic aneurysms 1990 to 2010. Glob. Heart. 2014;9:159–170. doi: 10.1016/j.gheart.2013.12.009. - DOI - PubMed
-
- DeMartino R.R., Sen I., Huang Y., Bower T.C., Oderich G.S., Pochettino A., Greason K., Kalra M., Johnstone J., Shuja F., et al. Population-Based Assessment of the Incidence of Aortic Dissection, Intramural Hematoma, and Penetrating Ulcer, and Its Associated Mortality From 1995 to 2015. Circ. Cardiovasc. Qual. Outcomes. 2018;11:e004689. doi: 10.1161/CIRCOUTCOMES.118.004689. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

