Cell Sources for Cartilage Repair-Biological and Clinical Perspective
- PMID: 34572145
- PMCID: PMC8468484
- DOI: 10.3390/cells10092496
Cell Sources for Cartilage Repair-Biological and Clinical Perspective
Abstract
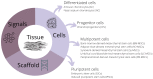
Cell-based therapy represents a promising treatment strategy for cartilage defects. Alone or in combination with scaffolds/biological signals, these strategies open many new avenues for cartilage tissue engineering. However, the choice of the optimal cell source is not that straightforward. Currently, various types of differentiated cells (articular and nasal chondrocytes) and stem cells (mesenchymal stem cells, induced pluripotent stem cells) are being researched to objectively assess their merits and disadvantages with respect to the ability to repair damaged articular cartilage. In this paper, we focus on the different cell types used in cartilage treatment, first from a biological scientist's perspective and then from a clinician's standpoint. We compare and analyze the advantages and disadvantages of these cell types and offer a potential outlook for future research and clinical application.
Keywords: articular cartilage; autologous chondrocyte transplantation; cartilage repair; chondrocytes; regenerative medicine; stem cells; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Mesenchymal stem cells for cartilage engineering.Biomed Mater Eng. 2012;22(1-3):69-80. doi: 10.3233/BME-2012-0691. Biomed Mater Eng. 2012. PMID: 22766704 Review.
-
Treatment of osteochondral defects in the rabbit's knee joint by implantation of allogeneic mesenchymal stem cells in fibrin clots.J Vis Exp. 2013 May 21;(75):e4423. doi: 10.3791/4423. J Vis Exp. 2013. PMID: 23728213 Free PMC article.
-
Cartilage Tissue Regeneration: The Roles of Cells, Stimulating Factors and Scaffolds.Curr Stem Cell Res Ther. 2018;13(7):547-567. doi: 10.2174/1574888X12666170608080722. Curr Stem Cell Res Ther. 2018. PMID: 28595567 Review.
-
Mesenchymal Stem/Progenitor Cells Derived from Articular Cartilage, Synovial Membrane and Synovial Fluid for Cartilage Regeneration: Current Status and Future Perspectives.Stem Cell Rev Rep. 2017 Oct;13(5):575-586. doi: 10.1007/s12015-017-9753-1. Stem Cell Rev Rep. 2017. PMID: 28721683 Review.
-
Different Sources of Stem Cells and their Application in Cartilage Tissue Engineering.Curr Stem Cell Res Ther. 2018;13(7):568-575. doi: 10.2174/1574888X13666180122151909. Curr Stem Cell Res Ther. 2018. PMID: 29359678 Review.
Cited by
-
Physical, Mechanical, and Biological Properties of Fibrin Scaffolds for Cartilage Repair.Int J Mol Sci. 2022 Aug 30;23(17):9879. doi: 10.3390/ijms23179879. Int J Mol Sci. 2022. PMID: 36077276 Free PMC article. Review.
-
Electrical Stimulation in Cartilage Tissue Engineering.Bioengineering (Basel). 2023 Apr 7;10(4):454. doi: 10.3390/bioengineering10040454. Bioengineering (Basel). 2023. PMID: 37106641 Free PMC article. Review.
-
Osteochondral organoids: current advances, applications, and upcoming challenges.Stem Cell Res Ther. 2024 Jun 21;15(1):183. doi: 10.1186/s13287-024-03790-5. Stem Cell Res Ther. 2024. PMID: 38902814 Free PMC article. Review.
-
Targeting the reorganization of F-actin for cell-based implantation cartilage repair therapies.Differentiation. 2025 May-Jun;143:100847. doi: 10.1016/j.diff.2025.100847. Epub 2025 Mar 1. Differentiation. 2025. PMID: 40068531 Review.
-
Trends in Cartilage Repair Techniques for Chondral Defects in the Hip in Germany: An Epidemiological Analysis from 2006 to 2022.Life (Basel). 2024 Oct 3;14(10):1262. doi: 10.3390/life14101262. Life (Basel). 2024. PMID: 39459562 Free PMC article.
References
-
- Panek M., Marijanovic I., Ivkovic A. Stem cells in bone regeneration. Period. Biol. 2015;117:177–184. doi: 10.1016/j.ijom.2011.07.851. - DOI
-
- Huang H., Xu H., Zhang J. Cartilage Tissue Engineering and Regeneration Techniques. IntechOpen; London, UK: 2019. Current Tissue Engineering Approaches for Cartilage Regeneration.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

