Wharton's Jelly-Derived Mesenchymal Stem Cells Reduce Fibrosis in a Mouse Model of Duchenne Muscular Dystrophy by Upregulating microRNA 499
- PMID: 34572277
- PMCID: PMC8469349
- DOI: 10.3390/biomedicines9091089
Wharton's Jelly-Derived Mesenchymal Stem Cells Reduce Fibrosis in a Mouse Model of Duchenne Muscular Dystrophy by Upregulating microRNA 499
Abstract
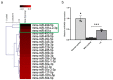
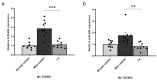
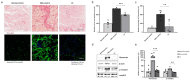
The aim of this study was to evaluate the therapeutic effects and mechanisms of Wharton's jelly-derived mesenchymal stem cells (WJ-MSCs) in an animal model of Duchenne muscular dystrophy (DMD). Mdx mice (3-5 months old) were administered five different doses of WJ-MSCs through their tail veins. A week after injection, grip strength measurements, creatine kinase (CK) assays, immunohistochemistry, and western blots were performed for comparison between healthy mice, mdx control mice, and WJ-MSC-injected mdx mice. WJ-MSCs exerted dose-dependent multisystem therapeutic effects in mdx mice, by decreasing CK, recovering normal behavior, regenerating muscle, and reducing apoptosis and fibrosis in skeletal muscle. We also confirmed that miR-499-5p is significantly downregulated in mdx mice, and that intravenous injection of WJ-MSCs enhanced its expression, leading to anti-fibrotic effects via targeting TGFβR 1 and 3. Thus, WJ-MSCs may represent novel allogeneic "off-the-shelf" cellular products for the treatment of DMD and possibly other muscle disorders.
Keywords: Duchenne muscular dystrophy; Wharton’s jelly-derived mesenchymal stem cell; microRNA-499-5p; skeletal muscle fibrosis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




 : block,
: block,  : inactivation).
: inactivation).References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

