Betulinic Acid Decorated with Polar Groups and Blue Emitting BODIPY Dye: Synthesis, Cytotoxicity, Cell-Cycle Analysis and Anti-HIV Profiling
- PMID: 34572290
- PMCID: PMC8472287
- DOI: 10.3390/biomedicines9091104
Betulinic Acid Decorated with Polar Groups and Blue Emitting BODIPY Dye: Synthesis, Cytotoxicity, Cell-Cycle Analysis and Anti-HIV Profiling
Abstract
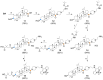
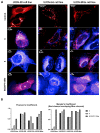
Betulinic acid (BA) is a potent triterpene, which has shown promising potential in cancer and HIV-1 treatment. Here, we report a synthesis and biological evaluation of 17 new compounds, including BODIPY labelled analogues derived from BA. The analogues terminated by amino moiety showed increased cytotoxicity (e.g., BA had on CCRF-CEM IC50 > 50 μM, amine 3 IC50 0.21 and amine 14 IC50 0.29). The cell-cycle arrest was evaluated and did not show general features for all the tested compounds. A fluorescence microscopy study of six derivatives revealed that only 4 and 6 were detected in living cells. These compounds were colocalized with the endoplasmic reticulum and mitochondria, indicating possible targets in these organelles. The study of anti-HIV-1 activity showed that 8, 10, 16, 17 and 18 have had IC50i > 10 μM. Only completely processed p24 CA was identified in the viruses formed in the presence of compounds 4 and 12. In the cases of 2, 8, 9, 10, 16, 17 and 18, we identified not fully processed p24 CA and p25 CA-SP1 protein. This observation suggests a similar mechanism of inhibition as described for bevirimat.
Keywords: BODIPY; betulinic acid; bevirimat; cancer; cell-cycle; cytotoxicity; fluorescent microscopy; maturation inhibitor.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

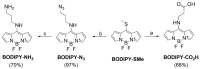


References
-
- Pisha E., Chai H., Lee I.S., Chagwedera T.E., Farnsworth N.R., Cordell G.A., Beecher C.W.W., Fong H.H.S., Kinghorn A.D., Brown D.M., et al. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat. Med. 1995;1:1046–1051. doi: 10.1038/nm1095-1046. - DOI - PubMed
-
- Fujioka T., Kashiwada Y., Kilkuskie R.E., Cosentino L.M., Ballas L.M., Jiang J.B., Janzen W.P., Chen I.-S., Lee K.-H. Anti-AIDS agents, 11. Betulinic acid and platanic acid as anti-HIV principles from Syzigium claviflorum, and the anti-HIV activity of structurally related triterpenoids. J. Nat. Prod. 1994;57:243–247. doi: 10.1021/np50104a008. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

