Heteronemin Suppresses Lymphangiogenesis through ARF-1 and MMP-9/VE-Cadherin/Vimentin
- PMID: 34572295
- PMCID: PMC8471334
- DOI: 10.3390/biomedicines9091109
Heteronemin Suppresses Lymphangiogenesis through ARF-1 and MMP-9/VE-Cadherin/Vimentin
Erratum in
-
Correction: Chen et al. Heteronemin Suppresses Lymphangiogenesis Through ARF-1 and MMP-9/VE-Cadherin/Vimentin. Biomedicines 2021, 9, 1109.Biomedicines. 2024 Nov 15;12(11):2609. doi: 10.3390/biomedicines12112609. Biomedicines. 2024. PMID: 39595215 Free PMC article.
Abstract
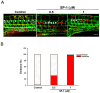
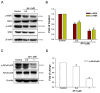
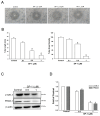
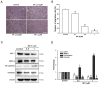
Lymphatic metastasis is a biological procedure associated with the pathogenesis of several diseases, especially in tumor metastasis. Therefore, regulation of lymphangiogenesis has become a promising strategy for cancer therapy. In this study, we aimed to investigate the anti-lymphangiogenic effect of heteronemin (SP-1) isolated from the sponge Hyrtios sp. in vitro and in vivo. Human lymphatic endothelial cells (LECs) were utilized to evaluate the anti-lymphangiogenic effect of SP-1 in vitro. Molecular docking, western blotting, flow-cytometry, MTT and ELISA were performed to investigate the mechanism of action. For in vivo approaches, the transgenic (fli1:EGFP; gata1:DsRed) zebrafish and mouse ear sponges were used. Molecular docking studies showed that SP-1 is a potent vascular endothelial growth factor receptor 3 (VEGFR-3)-binding compound. Treatment of LEC with SP-1 reduced the phosphorylation of VEGFR-3. SP-1 suppressed the development of the thoracic duct in zebrafish and mouse lymphangiogenesis ear sponges in vivo. Mechanistically, SP-1 induced the cell cycle arrest of LECs in the G0/G1 phase and reduced the downstream of VEGFR-3, such as phosphorylated MEK/ERK and NF-κB. In addition, SP-1 inhibited LECs' tubulogenesis and migration through the ARF-1 and MMP-9/VE-cadherin/vimentin. Overall, anti-lymphangiogenic properties of SP-1 occur by downregulating the VEGFR-3 cascade, ARF-1 and MMP-9/VE-cadherin/vimentin. Collectively, these results proposed that SP-1 might be a potential candidate for the treatment of lymphangiogenesis-associated diseases.
Keywords: ARF-1; MMP-9/VE-cadherin/vimentin; endothelial-to-mesenchymal transition; heteronemin; lymphangiogenesis; zebrafish.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

