Novel Coagulation Factor VIII Gene Therapy in a Mouse Model of Hemophilia A by Lipid-Coated Fe3O4 Nanoparticles
- PMID: 34572302
- PMCID: PMC8464966
- DOI: 10.3390/biomedicines9091116
Novel Coagulation Factor VIII Gene Therapy in a Mouse Model of Hemophilia A by Lipid-Coated Fe3O4 Nanoparticles
Abstract
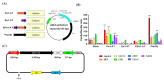
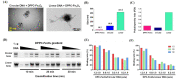
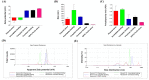
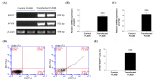
Hemophilia A is a bleeding disease caused by loss of coagulation factor VIII (FVIII) function. Although prophylactic FVIII infusion prevents abnormal bleeding, disability and joint damage in hemophilia patients are common. The cost of treatment is among the highest for a single disease, and the adverse effects of repeated infusion are still an issue that has not been addressed. In this study, we established a nonviral gene therapy strategy to treat FVIII knockout (FVIII KO) mice. A novel gene therapy approach was developed using dipalmitoylphosphatidylcholine formulated with iron oxide (DPPC-Fe3O4) to carry the B-domain-deleted (BDD)-FVIII plasmid, which was delivered into the FVIII KO mice via tail vein injection. Here, a liver-specific albumin promoter-driven BDD-FVIII plasmid was constructed, and the binding ability of circular DNA was confirmed to be more stable than that of linear DNA when combined with DPPC-Fe3O4 nanoparticles. The FVIII KO mice that received the DPPC-Fe3O4 plasmid complex were assessed by staining the ferric ion of DPPC-Fe3O4 nanoparticles with Prussian blue in liver tissue. The bleeding of the FVIII KO mice was improved in a few weeks, as shown by assessing the activated partial thromboplastin time (aPTT). Furthermore, no liver toxicity, thromboses, deaths, or persistent changes after nonviral gene therapy were found, as shown by serum liver indices and histopathology. The results suggest that this novel gene therapy can successfully improve hemostasis disorder in FVIII KO mice and might be a promising approach to treating hemophilia A patients in clinical settings.
Keywords: DPPC-Fe3O4; coagulation FVIII; gene therapy; hemophilia A mouse; nanoparticle.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

