Targeted Inhibition of Anti-Inflammatory Regulator Nrf2 Results in Breast Cancer Retardation In Vitro and In Vivo
- PMID: 34572304
- PMCID: PMC8471069
- DOI: 10.3390/biomedicines9091119
Targeted Inhibition of Anti-Inflammatory Regulator Nrf2 Results in Breast Cancer Retardation In Vitro and In Vivo
Abstract
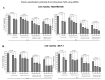
Nuclear factor erythroid-2 related factor-2 (Nrf2) is an oxidative stress-response transcriptional activator that promotes carcinogenesis through metabolic reprogramming, tumor promoting inflammation, and therapeutic resistance. However, the extension of Nrf2 expression and its involvement in regulation of breast cancer (BC) responses to chemotherapy remain largely unclear. This study determined the expression of Nrf2 in BC tissues (n = 46) and cell lines (MDA-MB-453, MCF-7, MDA-MB-231, MDA-MB-468) with diverse phenotypes. Immunohistochemical (IHC)analysis indicated lower Nrf2 expression in normal breast tissues, compared to BC samples, although the difference was not found to be significant. However, pharmacological inhibition and siRNA-induced downregulation of Nrf2 were marked by decreased activity of NADPH quinone oxidoreductase 1 (NQO1), a direct target of Nrf2. Silenced or inhibited Nrf2 signaling resulted in reduced BC proliferation and migration, cell cycle arrest, activation of apoptosis, and sensitization of BC cells to cisplatin in vitro. Ehrlich Ascites Carcinoma (EAC) cells demonstrated elevated levels of Nrf2 and were further tested in experimental mouse models in vivo. Intraperitoneal administration of pharmacological Nrf2 inhibitor brusatol slowed tumor cell growth. Brusatol increased lymphocyte trafficking towards engrafted tumor tissue in vivo, suggesting activation of anti-cancer effects in tumor microenvironment. Further large-scale BC testing is needed to confirm Nrf2 marker and therapeutic capacities for chemo sensitization in drug resistant and advanced tumors.
Keywords: Ehrlich Ascites Carcinoma cells; Nrf2; breast cancer; brusatol; chemo sensitization; tumorigenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

