Neural Precursor Cells Expanded Inside the 3D Micro-Scaffold Nichoid Present Different Non-Coding RNAs Profiles and Transcript Isoforms Expression: Possible Epigenetic Modulation by 3D Growth
- PMID: 34572306
- PMCID: PMC8472193
- DOI: 10.3390/biomedicines9091120
Neural Precursor Cells Expanded Inside the 3D Micro-Scaffold Nichoid Present Different Non-Coding RNAs Profiles and Transcript Isoforms Expression: Possible Epigenetic Modulation by 3D Growth
Abstract
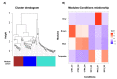
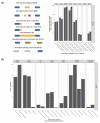
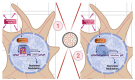
Non-coding RNAs show relevant implications in various biological and pathological processes. Thus, understanding the biological implications of these molecules in stem cell biology still represents a major challenge. The aim of this work is to study the transcriptional dysregulation of 357 non-coding genes, found through RNA-Seq approach, in murine neural precursor cells expanded inside the 3D micro-scaffold Nichoid versus standard culture conditions. Through weighted co-expression network analysis and functional enrichment, we highlight the role of non-coding RNAs in altering the expression of coding genes involved in mechanotransduction, stemness, and neural differentiation. Moreover, as non-coding RNAs are poorly conserved between species, we focus on those with human homologue sequences, performing further computational characterization. Lastly, we looked for isoform switching as possible mechanism in altering coding and non-coding gene expression. Our results provide a comprehensive dissection of the 3D scaffold Nichoid's influence on the biological and genetic response of neural precursor cells. These findings shed light on the possible role of non-coding RNAs in 3D cell growth, indicating that also non-coding RNAs are implicated in cellular response to mechanical stimuli.
Keywords: 3D-microscaffold; Nichoid; RNA interactions; RNA-seq; alternative splicing; isoform switching; mechanotransduction; non-coding RNAs.
Conflict of interest statement
MTR is a co-founder of the spin-off company MOAB Srl and holds shares.
Figures






Similar articles
-
Dissecting the Effect of a 3D Microscaffold on the Transcriptome of Neural Stem Cells with Computational Approaches: A Focus on Mechanotransduction.Int J Mol Sci. 2020 Sep 15;21(18):6775. doi: 10.3390/ijms21186775. Int J Mol Sci. 2020. PMID: 32942778 Free PMC article.
-
Neural precursors cells expanded in a 3D micro-engineered niche present enhanced therapeutic efficacy in vivo.Nanotheranostics. 2021 Jan 1;5(1):8-26. doi: 10.7150/ntno.50633. eCollection 2021. Nanotheranostics. 2021. PMID: 33391972 Free PMC article.
-
Bone Marrow Mesenchymal Stem Cells Expanded Inside the Nichoid Micro-Scaffold: a Focus on Anti-Inflammatory Response.Regen Eng Transl Med. 2023 Mar 20:1-12. doi: 10.1007/s40883-023-00296-z. Online ahead of print. Regen Eng Transl Med. 2023. PMID: 37363698 Free PMC article.
-
Non-coding RNAs: an emerging player in DNA damage response.Mutat Res Rev Mutat Res. 2015 Jan-Mar;763:202-11. doi: 10.1016/j.mrrev.2014.11.003. Epub 2014 Nov 13. Mutat Res Rev Mutat Res. 2015. PMID: 25795121 Review.
-
Drug-induced modifications and modulations of microRNAs and long non-coding RNAs for future therapy against Glioblastoma Multiforme.Gene. 2020 Jan 10;723:144126. doi: 10.1016/j.gene.2019.144126. Epub 2019 Oct 4. Gene. 2020. PMID: 31589963 Review.
Cited by
-
Study of lncRNAs in Pediatric Neurological Diseases: Methods, Analysis of the State-of-Art and Possible Therapeutic Implications.Pharmaceuticals (Basel). 2023 Nov 16;16(11):1616. doi: 10.3390/ph16111616. Pharmaceuticals (Basel). 2023. PMID: 38004481 Free PMC article. Review.
-
Retinal organoids and microfluidic chip-based approaches to explore the retinitis pigmentosa with USH2A mutations.Front Bioeng Biotechnol. 2022 Sep 14;10:939774. doi: 10.3389/fbioe.2022.939774. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36185441 Free PMC article.
-
Functional analysis and transcriptome profile of meninges and skin fibroblasts from human-aged donors.Cell Prolif. 2024 Aug;57(8):e13627. doi: 10.1111/cpr.13627. Epub 2024 Feb 29. Cell Prolif. 2024. PMID: 38421110 Free PMC article.
-
3D photopolymerized microstructured scaffolds influence nuclear deformation, nucleo/cytoskeletal protein organization, and gene regulation in mesenchymal stem cells.APL Bioeng. 2023 Sep 7;7(3):036112. doi: 10.1063/5.0153215. eCollection 2023 Sep. APL Bioeng. 2023. PMID: 37692376 Free PMC article.

