Collagen Bioinks for Bioprinting: A Systematic Review of Hydrogel Properties, Bioprinting Parameters, Protocols, and Bioprinted Structure Characteristics
- PMID: 34572322
- PMCID: PMC8468019
- DOI: 10.3390/biomedicines9091137
Collagen Bioinks for Bioprinting: A Systematic Review of Hydrogel Properties, Bioprinting Parameters, Protocols, and Bioprinted Structure Characteristics
Abstract
Bioprinting is a modern tool suitable for creating cell scaffolds and tissue or organ carriers from polymers that mimic tissue properties and create a natural environment for cell development. A wide range of polymers, both natural and synthetic, are used, including extracellular matrix and collagen-based polymers. Bioprinting technologies, based on syringe deposition or laser technologies, are optimal tools for creating precise constructs precisely from the combination of collagen hydrogel and cells. This review describes the different stages of bioprinting, from the extraction of collagen hydrogels and bioink preparation, over the parameters of the printing itself, to the final testing of the constructs. This study mainly focuses on the use of physically crosslinked high-concentrated collagen hydrogels, which represents the optimal way to create a biocompatible 3D construct with sufficient stiffness. The cell viability in these gels is mainly influenced by the composition of the bioink and the parameters of the bioprinting process itself (temperature, pressure, cell density, etc.). In addition, a detailed table is included that lists the bioprinting parameters and composition of custom bioinks from current studies focusing on printing collagen gels without the addition of other polymers. Last but not least, our work also tries to refute the often-mentioned fact that highly concentrated collagen hydrogel is not suitable for 3D bioprinting and cell growth and development.
Keywords: bioink; bioprinting; bioprinting parameters; collagen; hydrogel; hydrogel properties.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

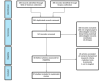





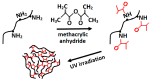
Similar articles
-
A hydrogel bioink toolkit for mimicking native tissue biochemical and mechanical properties in bioprinted tissue constructs.Acta Biomater. 2015 Oct;25:24-34. doi: 10.1016/j.actbio.2015.07.030. Epub 2015 Jul 22. Acta Biomater. 2015. PMID: 26210285
-
Advancing bioinks for 3D bioprinting using reactive fillers: A review.Acta Biomater. 2020 Sep 1;113:1-22. doi: 10.1016/j.actbio.2020.06.040. Epub 2020 Jul 2. Acta Biomater. 2020. PMID: 32622053 Review.
-
pH Modification of High-Concentrated Collagen Bioinks as a Factor Affecting Cell Viability, Mechanical Properties, and Printability.Gels. 2021 Dec 7;7(4):252. doi: 10.3390/gels7040252. Gels. 2021. PMID: 34940312 Free PMC article.
-
Employing PEG crosslinkers to optimize cell viability in gel phase bioinks and tailor post printing mechanical properties.Acta Biomater. 2019 Nov;99:121-132. doi: 10.1016/j.actbio.2019.09.007. Epub 2019 Sep 17. Acta Biomater. 2019. PMID: 31539655
-
Hydrogel Bioink Reinforcement for Additive Manufacturing: A Focused Review of Emerging Strategies.Adv Mater. 2020 Jan;32(1):e1902026. doi: 10.1002/adma.201902026. Epub 2019 Oct 10. Adv Mater. 2020. PMID: 31599073 Review.
Cited by
-
Experimental Study on Compatibility of Human Bronchial Epithelial Cells in Collagen-Alginate Bioink for 3D Printing.Bioengineering (Basel). 2024 Aug 23;11(9):862. doi: 10.3390/bioengineering11090862. Bioengineering (Basel). 2024. PMID: 39329604 Free PMC article.
-
Recent development in multizonal scaffolds for osteochondral regeneration.Bioact Mater. 2023 Feb 2;25:122-159. doi: 10.1016/j.bioactmat.2023.01.012. eCollection 2023 Jul. Bioact Mater. 2023. PMID: 36817819 Free PMC article. Review.
-
Interpenetrating networks of fibrillar and amorphous collagen promote cell spreading and hydrogel stability.Acta Biomater. 2025 Jan 24;193:128-142. doi: 10.1016/j.actbio.2025.01.009. Epub 2025 Jan 9. Acta Biomater. 2025. PMID: 39798635
-
Advancements in 3D skin bioprinting: processes, bioinks, applications and sensor integration.Int J Extrem Manuf. 2025 Feb 1;7(1):012009. doi: 10.1088/2631-7990/ad878c. Epub 2024 Nov 19. Int J Extrem Manuf. 2025. PMID: 39569402 Free PMC article. Review.
-
Head and Neck 3D Bioprinting-A Review on Recent Advancements in Soft Tissue 3D Bioprinting and Medical Applications.J Funct Biomater. 2025 Jun 30;16(7):240. doi: 10.3390/jfb16070240. J Funct Biomater. 2025. PMID: 40710454 Free PMC article. Review.
References
-
- Silva L.P. 3D and 4D Printing in Biomedical Applications. Wiley; New York, NY, USA: 2019. Current Trends and Challenges in Biofabrication Using Biomaterials and Nanomaterials: Future Perspectives for 3D/4D Bioprinting; pp. 373–421. - DOI
-
- Moldovan F. Recent Trends in Bioprinting. Procedia Manuf. 2019;32:95–101. doi: 10.1016/j.promfg.2019.02.188. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

