Effective Accentuation of Voltage-Gated Sodium Current Caused by Apocynin (4'-Hydroxy-3'-methoxyacetophenone), a Known NADPH-Oxidase Inhibitor
- PMID: 34572332
- PMCID: PMC8464932
- DOI: 10.3390/biomedicines9091146
Effective Accentuation of Voltage-Gated Sodium Current Caused by Apocynin (4'-Hydroxy-3'-methoxyacetophenone), a Known NADPH-Oxidase Inhibitor
Abstract
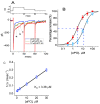
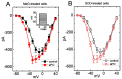
Apocynin (aPO, 4'-Hydroxy-3'-methoxyacetophenone) is a cell-permeable, anti-inflammatory phenolic compound that acts as an inhibitor of NADPH-dependent oxidase (NOX). However, the mechanisms through which aPO can interact directly with plasmalemmal ionic channels to perturb the amplitude or gating of ionic currents in excitable cells remain incompletely understood. Herein, we aimed to investigate any modifications of aPO on ionic currents in pituitary GH3 cells or murine HL-1 cardiomyocytes. In whole-cell current recordings, GH3-cell exposure to aPO effectively stimulated the peak and late components of voltage-gated Na+ current (INa) with different potencies. The EC50 value of aPO required for its differential increase in peak or late INa in GH3 cells was estimated to be 13.2 or 2.8 μM, respectively, whereas the KD value required for its retardation in the slow component of current inactivation was 3.4 μM. The current-voltage relation of INa was shifted slightly to more negative potential during cell exposure to aPO (10 μM); however, the steady-state inactivation curve of the current was shifted in a rightward direction in its presence. Recovery of peak INa inactivation was increased in the presence of 10 μM aPO. In continued presence of aPO, further application of rufinamide or ranolazine attenuated aPO-stimulated INa. In methylglyoxal- or superoxide dismutase-treated cells, the stimulatory effect of aPO on peak INa remained effective. By using upright isosceles-triangular ramp pulse of varying duration, the amplitude of persistent INa measured at low or high threshold was enhanced by the aPO presence, along with increased hysteretic strength appearing at low or high threshold. The addition of aPO (10 μM) mildly inhibited the amplitude of erg-mediated K+ current. Likewise, in HL-1 murine cardiomyocytes, the aPO presence increased the peak amplitude of INa as well as decreased the inactivation or deactivation rate of the current, and further addition of ranolazine or esaxerenone attenuated aPO-accentuated INa. Altogether, this study provides a distinctive yet unidentified finding that, despite its effectiveness in suppressing NOX activity, aPO may directly and concertedly perturb the amplitude, gating and voltage-dependent hysteresis of INa in electrically excitable cells. The interaction of aPO with ionic currents may, at least in part, contribute to the underlying mechanisms through which it affects neuroendocrine, endocrine or cardiac function.
Keywords: NADPH-dependent oxidase (NOX); apocynin (4′-Hydroxy-3′-methoxyacetophenone); current kinetics; electrically excitable cell; erg-mediated K+ current; persistent Na+ current; voltage-dependent hysteresis; voltage-gated Na+ current.
Conflict of interest statement
The authors declare no competing interests that are directly relevant to the present study.
Figures







Similar articles
-
Recent Advances in Ionic Mechanisms in Pituitary Cells: Implications for Electrophysiological and Electropharmacological Research.J Clin Med. 2025 Apr 30;14(9):3117. doi: 10.3390/jcm14093117. J Clin Med. 2025. PMID: 40364147 Free PMC article. Review.
-
Characterization of Direct Perturbations on Voltage-Gated Sodium Current by Esaxerenone, a Nonsteroidal Mineralocorticoid Receptor Blocker.Biomedicines. 2021 May 13;9(5):549. doi: 10.3390/biomedicines9050549. Biomedicines. 2021. PMID: 34068333 Free PMC article.
-
The Evidence for Sparsentan-Mediated Inhibition of INa and IK(erg): Possibly Unlinked to Its Antagonism of Angiotensin II or Endothelin Type a Receptor.Biomedicines. 2021 Dec 31;10(1):86. doi: 10.3390/biomedicines10010086. Biomedicines. 2021. PMID: 35052766 Free PMC article.
-
Effectiveness of Columbianadin, a Bioactive Coumarin Derivative, in Perturbing Transient and Persistent INa.Int J Mol Sci. 2021 Jan 9;22(2):621. doi: 10.3390/ijms22020621. Int J Mol Sci. 2021. PMID: 33435511 Free PMC article.
-
Effective Perturbations by Small-Molecule Modulators on Voltage-Dependent Hysteresis of Transmembrane Ionic Currents.Int J Mol Sci. 2022 Aug 21;23(16):9453. doi: 10.3390/ijms23169453. Int J Mol Sci. 2022. PMID: 36012718 Free PMC article. Review.
Cited by
-
Inhibitory Effectiveness in Delayed-Rectifier Potassium Current Caused by Vortioxetine, Known to Be a Novel Antidepressant.Biomedicines. 2022 Jun 3;10(6):1318. doi: 10.3390/biomedicines10061318. Biomedicines. 2022. PMID: 35740340 Free PMC article.
-
Investigating the Impact of Selective Modulators on the Renin-Angiotensin-Aldosterone System: Unraveling Their Off-Target Perturbations of Transmembrane Ionic Currents.Int J Mol Sci. 2023 Sep 12;24(18):14007. doi: 10.3390/ijms241814007. Int J Mol Sci. 2023. PMID: 37762309 Free PMC article. Review.
-
Recent Advances in Ionic Mechanisms in Pituitary Cells: Implications for Electrophysiological and Electropharmacological Research.J Clin Med. 2025 Apr 30;14(9):3117. doi: 10.3390/jcm14093117. J Clin Med. 2025. PMID: 40364147 Free PMC article. Review.
-
Characterization in Inhibitory Effectiveness of Carbamazepine in Voltage-Gated Na+ and Erg-Mediated K+ Currents in a Mouse Neural Crest-Derived (Neuro-2a) Cell Line.Int J Mol Sci. 2022 Jul 17;23(14):7892. doi: 10.3390/ijms23147892. Int J Mol Sci. 2022. PMID: 35887240 Free PMC article.
-
Characterization in Effective Stimulation on the Magnitude, Gating, Frequency Dependence, and Hysteresis of INa Exerted by Picaridin (or Icaridin), a Known Insect Repellent.Int J Mol Sci. 2022 Aug 26;23(17):9696. doi: 10.3390/ijms23179696. Int J Mol Sci. 2022. PMID: 36077093 Free PMC article.
References
-
- Du Z.-D., Yu S., Qi Y., Qu T.-F., He L., Wei W., Liu K., Gong S.-S. NADPH oxidase inhibitor apocynin decreases mitochondrial dysfunction and apoptosis in the ventral cochlear nucleus of D-galactose-induced aging model in rats. Neurochem. Int. 2019;124:31–40. doi: 10.1016/j.neuint.2018.12.008. - DOI - PubMed
LinkOut - more resources
Full Text Sources

