Mitoxantrone Shows In Vitro, but Not In Vivo Antiviral Activity against Human Respiratory Syncytial Virus
- PMID: 34572362
- PMCID: PMC8472696
- DOI: 10.3390/biomedicines9091176
Mitoxantrone Shows In Vitro, but Not In Vivo Antiviral Activity against Human Respiratory Syncytial Virus
Abstract
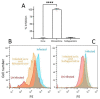
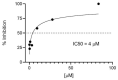
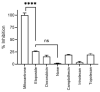
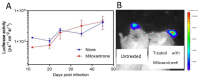
Human respiratory syncytial virus (HRSV) is the most common cause of severe respiratory infections in infants and young children, often leading to hospitalization. In addition, this virus poses a serious health risk in immunocompromised individuals and the elderly. HRSV is also a major nosocomial hazard in healthcare service units for patients of all ages. Therefore, the development of antiviral treatments against HRSV is a global health priority. In this study, mitoxantrone, a synthetic anthraquinone with previously reported in vitro antiprotozoal and antiviral activities, inhibits HRSV replication in vitro, but not in vivo in a mice model. These results have implications for preclinical studies of some drug candidates.
Keywords: HRSV; antivirals; bioluminescence; drugs.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Chloroquine and pyrimethamine inhibit the replication of human respiratory syncytial virus A.J Gen Virol. 2021 Aug;102(8). doi: 10.1099/jgv.0.001627. J Gen Virol. 2021. PMID: 34342560
-
Establishment of a lethal aged mouse model of human respiratory syncytial virus infection.Antiviral Res. 2019 Jan;161:125-133. doi: 10.1016/j.antiviral.2018.11.015. Epub 2018 Nov 29. Antiviral Res. 2019. PMID: 30503888
-
Novel therapies and vaccines against the human respiratory syncytial virus.Expert Opin Investig Drugs. 2015;24(12):1613-30. doi: 10.1517/13543784.2015.1099626. Epub 2015 Oct 12. Expert Opin Investig Drugs. 2015. PMID: 26457559 Review.
-
Hospital outbreak of human respiratory syncytial virus (HRSV) illness in immunocompromised hospitalized children during summer.Clin Respir J. 2015 Apr;9(2):180-4. doi: 10.1111/crj.12121. Epub 2014 Mar 6. Clin Respir J. 2015. PMID: 24521518
-
Contribution of Fcγ Receptor-Mediated Immunity to the Pathogenesis Caused by the Human Respiratory Syncytial Virus.Front Cell Infect Microbiol. 2019 Mar 29;9:75. doi: 10.3389/fcimb.2019.00075. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 30984626 Free PMC article. Review.
Cited by
-
Current Potential Therapeutic Approaches against SARS-CoV-2: A Review.Biomedicines. 2021 Nov 4;9(11):1620. doi: 10.3390/biomedicines9111620. Biomedicines. 2021. PMID: 34829850 Free PMC article. Review.
References
-
- Collins P.L., Chanock R.M., Murphy B.R. Respiratory Syncytial Virus. In: Knipe D.M., Howley P.M., editors. Fields Virology. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2013. pp. 1086–1124.
-
- Taniguchi A., Kawada J.I., Go K., Fujishiro N., Hosokawa Y., Maki Y., Sugiyama Y., Suzuki M., Tsuji T., Hoshino S., et al. Comparison of Clinical Characteristics of Human Metapneumovirus and Respiratory Syncytial Virus Infections in Hospitalized Young Children. Jpn. J. Infect. Dis. 2019;72:237–242. doi: 10.7883/yoken.JJID.2018.480. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

