Safety and Tolerability of the Adeno-Associated Virus Vector, AAV6.2FF, Expressing a Monoclonal Antibody in Murine and Ovine Animal Models
- PMID: 34572372
- PMCID: PMC8464737
- DOI: 10.3390/biomedicines9091186
Safety and Tolerability of the Adeno-Associated Virus Vector, AAV6.2FF, Expressing a Monoclonal Antibody in Murine and Ovine Animal Models
Abstract
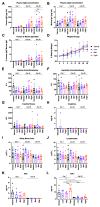
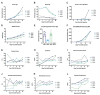
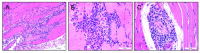
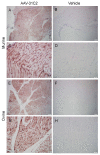
Adeno-associated virus (AAV) vector mediated expression of therapeutic monoclonal antibodies is an alternative strategy to traditional vaccination to generate immunity in immunosuppressed or immunosenescent individuals. In this study, we vectorized a human monoclonal antibody (31C2) directed against the spike protein of SARS-CoV-2 and determined the safety profile of this AAV vector in mice and sheep as a large animal model. In both studies, plasma biochemical parameters and hematology were comparable to untreated controls. Except for mild myositis at the site of injection, none of the major organs revealed any signs of toxicity. AAV-mediated human IgG expression increased steadily throughout the 28-day study in sheep, resulting in peak concentrations of 21.4-46.7 µg/ mL, demonstrating practical scale up from rodent to large animal models. This alternative approach to immunity is worth further exploration after this demonstration of safety, tolerability, and scalability in a large animal model.
Keywords: adeno-associated virus (AAV) vector; large animal model; monoclonal antibody; safety; tolerability; vectored immunoprophylaxis.
Conflict of interest statement
LPvL and SKW are inventors on a US patent for the AAV6.2FF capsid. This patent (US20190216949) is licensed to Avamab Pharma Inc., where BT, LPvL and SKW are co-founders and BT serves as an executive. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Adeno-associated virus mediated expression of monoclonal antibody MR191 protects mice against Marburg virus and provides long-term expression in sheep.Gene Ther. 2025 Jan;32(1):50-59. doi: 10.1038/s41434-022-00361-2. Epub 2022 Sep 1. Gene Ther. 2025. PMID: 36050451
-
AAV-monoclonal antibody expression protects mice from Ebola virus without impeding the endogenous antibody response to heterologous challenge.Mol Ther Methods Clin Dev. 2022 Aug 12;26:505-518. doi: 10.1016/j.omtm.2022.08.003. eCollection 2022 Sep 8. Mol Ther Methods Clin Dev. 2022. PMID: 36092367 Free PMC article.
-
A Novel Triple-Mutant AAV6 Capsid Induces Rapid and Potent Transgene Expression in the Muscle and Respiratory Tract of Mice.Mol Ther Methods Clin Dev. 2018 Apr 14;9:323-329. doi: 10.1016/j.omtm.2018.04.005. eCollection 2018 Jun 15. Mol Ther Methods Clin Dev. 2018. PMID: 30038936 Free PMC article.
-
AAV Vectored Immunoprophylaxis for Filovirus Infections.Trop Med Infect Dis. 2020 Nov 9;5(4):169. doi: 10.3390/tropicalmed5040169. Trop Med Infect Dis. 2020. PMID: 33182447 Free PMC article. Review.
-
Recent Advancements in AAV-Vectored Immunoprophylaxis in the Nonhuman Primate Model.Biomedicines. 2023 Aug 8;11(8):2223. doi: 10.3390/biomedicines11082223. Biomedicines. 2023. PMID: 37626720 Free PMC article. Review.
Cited by
-
AAV-vectored expression of monospecific or bispecific monoclonal antibodies protects mice from lethal Pseudomonas aeruginosa pneumonia.Gene Ther. 2024 Jul;31(7-8):400-412. doi: 10.1038/s41434-024-00453-1. Epub 2024 Apr 27. Gene Ther. 2024. PMID: 38678160
-
Single-shot AAV-vectored vaccine against SARS-CoV-2 with fast and long-lasting immunity.Acta Pharm Sin B. 2023 May;13(5):2219-2233. doi: 10.1016/j.apsb.2022.07.004. Epub 2022 Jul 12. Acta Pharm Sin B. 2023. PMID: 35846427 Free PMC article.
-
Antibody-based protection against respiratory syncytial virus in mice and their offspring through vectored immunoprophylaxis.Gene Ther. 2025 Jan;32(1):38-49. doi: 10.1038/s41434-023-00385-2. Epub 2023 Feb 2. Gene Ther. 2025. PMID: 36732618
-
Expanding the Reach of Monoclonal Antibodies: A Review of Synthetic Nucleic Acid Delivery in Immunotherapy.Antibodies (Basel). 2023 Jul 6;12(3):46. doi: 10.3390/antib12030046. Antibodies (Basel). 2023. PMID: 37489368 Free PMC article. Review.
-
Delayed viral vector mediated delivery of neurotrophin-3 improves skilled hindlimb function and stability after thoracic contusion.Exp Neurol. 2023 Feb;360:114278. doi: 10.1016/j.expneurol.2022.114278. Epub 2022 Nov 28. Exp Neurol. 2023. PMID: 36455639 Free PMC article.
References
-
- Olchanski N., Hansen R.N., Pope E., D’Cruz B., Fergie J., Goldstein M., Krilov L.R., McLaurin K.K., Nabrit-Stephens B., Oster G., et al. Palivizumab Prophylaxis for Respiratory Syncytial Virus: Examining the Evidence Around Value. Open Forum Infect. Dis. 2018;5:ofy031. doi: 10.1093/ofid/ofy031. - DOI - PMC - PubMed
-
- Zost S.J., Gilchuk P., Chen R.E., Case J.B., Reidy J.X., Trivette A., Nargi R.S., Sutton R.E., Suryadevara N., Chen E.C., et al. Rapid isolation and profiling of a diverse panel of human monoclonal antibodies targeting the SARS-CoV-2 spike protein. Nat. Med. 2020;26:1422–1427. doi: 10.1038/s41591-020-0998-x. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

