A Role of Stress Sensor Nrf2 in Stimulating Thermogenesis and Energy Expenditure
- PMID: 34572382
- PMCID: PMC8472024
- DOI: 10.3390/biomedicines9091196
A Role of Stress Sensor Nrf2 in Stimulating Thermogenesis and Energy Expenditure
Abstract
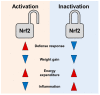
During chronic cold stress, thermogenic adipocytes generate heat through uncoupling of mitochondrial respiration from ATP synthesis. Recent discovery of various dietary phytochemicals, endogenous metabolites, synthetic compounds, and their molecular targets for stimulating thermogenesis has provided promising strategies to treat or prevent obesity and its associated metabolic diseases. Nuclear factor E2 p45-related factor 2 (Nrf2) is a stress response protein that plays an important role in obesity and metabolisms. However, both Nrf2 activation and Nrf2 inhibition can suppress obesity and metabolic diseases. Here, we summarized and discussed conflicting findings of Nrf2 activities accounting for part of the variance in thermogenesis and energy metabolism. We also discussed the utility of Nrf2-activating mechanisms for their potential applications in stimulating energy expenditure to prevent obesity and improve metabolic deficits.
Keywords: Nrf2; energy expenditure; metabolic diseases; obesity; thermogenesis; uncoupling.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

