Targeting Cancer Cells Overexpressing Folate Receptors with New Terpolymer-Based Nanocapsules: Toward a Novel Targeted DNA Delivery System for Cancer Therapy
- PMID: 34572461
- PMCID: PMC8471118
- DOI: 10.3390/biomedicines9091275
Targeting Cancer Cells Overexpressing Folate Receptors with New Terpolymer-Based Nanocapsules: Toward a Novel Targeted DNA Delivery System for Cancer Therapy
Abstract
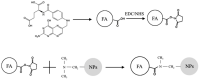
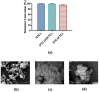
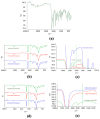
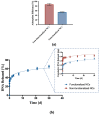
Chemotherapeutics represent the standard treatment for a wide range of cancers. However, these agents also affect healthy cells, thus leading to severe off-target effects. Given the non-selectivity of the commonly used drugs, any increase in the selective tumor tissue uptake would represent a significant improvement in cancer therapy. Recently, the use of gene therapy to completely remove the lesion and avoid the toxicity of chemotherapeutics has become a tendency in oncotherapy. Ideally, the genetic material must be safely transferred from the site of administration to the target cells, without involving healthy tissues. This can be achieved by encapsulating genes into non-viral carriers and modifying their surface with ligands with high selectivity and affinity for a relevant receptor on the target cells. Hence, in this work we evaluate the use of terpolymer-based nanocapsules for the targeted delivery of DNA toward cancer cells. The surface of the nanocapsules is decorated with folic acid to actively target the folate receptors overexpressed on a variety of cancer cells. The nanocapsules demonstrate a good ability of encapsulating and releasing DNA. Moreover, the presence of the targeting moieties on the surface of the nanocapsules favors cell uptake, opening up the possibility of more effective therapies.
Keywords: DNA delivery; active targeting; cancer; folic acid; gene therapy; nanocapsules; polymeric nanoparticles; terpolymer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Oil-encapsulating PEO-PPO-PEO/PEG shell cross-linked nanocapsules for target-specific delivery of paclitaxel.Biomacromolecules. 2007 Feb;8(2):650-6. doi: 10.1021/bm0608939. Biomacromolecules. 2007. PMID: 17291088
-
Clickable protein nanocapsules for targeted delivery of recombinant p53 protein.J Am Chem Soc. 2014 Oct 29;136(43):15319-25. doi: 10.1021/ja508083g. Epub 2014 Oct 17. J Am Chem Soc. 2014. PMID: 25289975
-
Folate-targeted Pluronic-chitosan nanocapsules loaded with IR780 for near-infrared fluorescence imaging and photothermal-photodynamic therapy of ovarian cancer.Colloids Surf B Biointerfaces. 2021 Jul;203:111755. doi: 10.1016/j.colsurfb.2021.111755. Epub 2021 Apr 8. Colloids Surf B Biointerfaces. 2021. PMID: 33862575
-
Discovery and development of folic-acid-based receptor targeting for imaging and therapy of cancer and inflammatory diseases.Acc Chem Res. 2008 Jan;41(1):120-9. doi: 10.1021/ar7000815. Epub 2007 Jul 27. Acc Chem Res. 2008. PMID: 17655275 Review.
-
Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy.Eur J Pharm Biopharm. 2015 Jun;93:52-79. doi: 10.1016/j.ejpb.2015.03.018. Epub 2015 Mar 23. Eur J Pharm Biopharm. 2015. PMID: 25813885 Review.
Cited by
-
Preparation and Evaluation of Folate Modified PEG-PLLA Nanoparticles Loaded with Lycorine for Glioma Treatment.Molecules. 2024 Feb 29;29(5):1081. doi: 10.3390/molecules29051081. Molecules. 2024. PMID: 38474593 Free PMC article.
-
Design of Chitosan-Coated, Quercetin-Loaded PLGA Nanoparticles for Enhanced PSMA-Specific Activity on LnCap Prostate Cancer Cells.Biomedicines. 2023 Apr 18;11(4):1201. doi: 10.3390/biomedicines11041201. Biomedicines. 2023. PMID: 37189819 Free PMC article.
-
Role of Cell Adhesion in Cancer Metastasis Formation: A Review.ACS Omega. 2025 Feb 9;10(6):5193-5213. doi: 10.1021/acsomega.4c08140. eCollection 2025 Feb 18. ACS Omega. 2025. PMID: 39989825 Free PMC article. Review.
-
Enhanced Uptake and Phototoxicity of C60@albumin Hybrids by Folate Bioconjugation.Nanomaterials (Basel). 2022 Oct 6;12(19):3501. doi: 10.3390/nano12193501. Nanomaterials (Basel). 2022. PMID: 36234629 Free PMC article.
-
Synthesis of Multifunctional Polymersomes Prepared by Polymerization-Induced Self-Assembly.Polymers (Basel). 2023 Jul 17;15(14):3070. doi: 10.3390/polym15143070. Polymers (Basel). 2023. PMID: 37514459 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

